CSL Behring Announces First Two Patients Treated with HEMGENIX® (etranacogene dezaparvovec) Gene The
MARBURG, Germany, July 04, 2024 (GLOBE NEWSWIRE) -- Global biotechnology leader CSL Behring (ASX: CSL) today announced that two hemophilia B patients were treated with the gene therapy HEMGENIX® (etranacogene dezaparvovec) at Hemophilia Treatment Centers in France. This milestone achievement makes HEMGENIX® the first gene therapy administered as a treatment in a real-world setting for hemophilia B in Europe.
HEMGENIX® is the first one-time gene therapy approved in Europe for the treatment of adults with severe and moderately severe hemophilia B, an inherited bleeding disorder caused by the lack of Factor IX (a protein needed to produce blood clots to stop bleeding). It is used in adults without a history of Factor IX inhibitors.1
Following European Commission approval, HEMGENIX® was the first ever therapy to be granted Direct Access in France2, thus enabling the first patients to be treated in Europe outside of the clinical program.
Though effective, current therapies can be time intensive and require regular treatment that can have a substantial impact on a patient’s daily life.3 HEMGENIX® offers a one-time treatment, allowing people living with hemophilia B to produce their own Factor IX, which can lower the risk of bleeding.4
“Only a few decades ago, gene therapy for hemophilia was a distant concept, which has now become reality. Accordingly, the first two patients treated with HEMGENIX® since receiving European approval is a major accomplishment and a testament to the joint commitment of the hemophilia B community, as well as the access and reimbursement authorities, in bringing innovative therapies to patients,” said Dr Lutz Bonacker SVP and General Manager, CSL Behring Commercial Operations Europe. “This milestone has been made possible by the innovative Direct Access scheme adopted in France, allowing patients to benefit from early access to pioneering treatments. We are encouraged to see increasing access to gene therapies in European countries and are fully committed to ensuring that access to potentially life-changing treatment continues.”
HEMGENIX® was granted conditional marketing authorisation by the European Commission (EC) for the European Union and European Economic Area in February 2023, following approval from the U.S. Food and Drug Administration (FDA) in November 2022. It has also been approved by Health Canada, the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA), Switzerland’s Swissmedic and Australia’s Therapeutic Goods Administration (TGA).
The multi-year clinical development of HEMGENIX® was led by uniQure and sponsorship of the clinical trials transitioned to CSL after it licensed global rights to commercialise the treatment.
About Hemophilia B
Hemophilia B is a life-threatening rare disease. People with the condition are particularly vulnerable to bleeds in their joints, muscles, and internal organs, leading to pain, swelling, and joint damage. Current treatments for moderate to severe hemophilia B include life-long prophylactic infusions of factor IX to temporarily replace or supplement low levels of the blood-clotting factor.
About HEMGENIX®
HEMGENIX® is a gene therapy that reduces the rate of abnormal bleeding in eligible people with hemophilia B by enabling the body to continuously produce factor IX, the deficient protein in hemophilia B. It uses AAV5, a non-infectious viral vector, called an adeno-associated virus (AAV). The AAV5 vector carries the Padua gene variant of Factor IX (FIX-Padua) to the target cells in the liver, generating factor IX proteins that are 5x-8x more active than normal. These genetic instructions remain in the target cells, but generally do not become a part of a person’s own DNA. Once delivered, the new genetic instructions allow the cellular machinery to produce stable levels of factor IX.
About the Pivotal HOPE-B Trial
The pivotal Phase III HOPE-B trial is an ongoing, multinational, open-label, single-arm study to evaluate the safety and efficacy of HEMGENIX®. Fifty-four adult hemophilia B patients classified as having moderately severe to severe hemophilia B and requiring prophylactic factor IX replacement therapy were enrolled in a prospective, six-month or longer observational period during which time they continued to use their current standard of care therapy to establish a baseline Annual Bleeding Rate (ABR). After the six-month lead-in period, patients received a single intravenous administration of HEMGENIX® at the 2x10^13 gc/kg dose. Patients were not excluded from the trial based on pre-existing neutralizing antibodies (NAbs) to AAV5.
A total of 54 patients received a single dose of HEMGENIX® in the pivotal trial, with 52 patients completing at least three years of follow-up. The primary endpoint in the pivotal HOPE-B study was ABR 52 weeks after achievement of stable factor IX expression (months 7 to 18) compared with the six-month lead-in period. For this endpoint, ABR was measured from month seven to month 18 after infusion, ensuring the observation period represented a steady-state factor IX transgene expression. Secondary endpoints included assessment of factor IX activity.
No serious treatment-related adverse reactions were reported. One death resulting from urosepsis and cardiogenic shock in a 77-year-old patient at 65 weeks following dosing was considered unrelated to treatment by investigators and the company sponsor. A serious adverse event of hepatocellular carcinoma was determined to be unrelated to treatment with HEMGENIX® by independent molecular tumour characterization and vector integration analysis. No inhibitors to factor IX were reported.
Long-term three-year data presented at the 17th Annual Congress of the European Association for Haemophilia and Allied Disorders (EAHAD) 2024 continue to reinforce the potential long-lasting efficacy and safety of HEMGENIX® and the ongoing benefit of this treatment for people living with hemophilia B.
About CSL
CSL (ASX:CSL; USOTC:CSLLY) is a global biotechnology company with a dynamic portfolio of lifesaving medicines, including those that treat hemophilia and immune deficiencies, vaccines to prevent influenza, and therapies in iron deficiency and nephrology. Since our start in 1916, we have been driven by our promise to save lives using the latest technologies. Today, CSL – including our three businesses: CSL Behring, CSL Seqirus and CSL Vifor – provides lifesaving products to patients in more than 100 countries and employs 32,000 people. Our unique combination of commercial strength, R&D focus and operational excellence enables us to identify, develop and deliver innovations so our patients can live life to the fullest. For inspiring stories about the promise of biotechnology, visit CSL.com/Vita. For more information about CSL, visit CSL.com.
Media Contacts
Stephanie Fuchs
Mobile: +49 151 584 388 60
Email: Stephanie.Fuchs@cslbehring.com
References
1 European Medicines Agency. First Gene therapy to treat haemophilia B. Available at: https://www.ema.europa.eu/en/news/first-gene-therapy-treat-haemophilia-b. [Accessed May 2024].
2 Republique Française. Légifrance: Article 62 of Law No. 2021-1754. Available at: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000048551003 [Accessed May 2024].
3 Leebeek, F & Miesbach, W. (2021) Gene therapy for haemophilia: a review on clinical benefit, limitations, and remaining issues. Blood. Vol 138, Issue 11. pp923-931.
4 Coppens M et al. Etranacogene dezaparvovec gene therapy for haemophilia B (HOPE-B): 24-month post-hoc efficacy and safety data from a single-arm, multicentre, phase 3 trial. The Lancet Haematology 2024; 11(4):E265-E275.
- 临商银行半程支行上门服务获好评
- 直流dc500v550v600v转变ac220V正弦波逆变器
- 500辆九识智能产品在如皋“持证上岗”
- 中信银行“少年看中国”主题活动2024乘风开启
- 馨悦采耳养生保健馆:舒适环境,专业服务,打造全新养生体验!
- 中国人寿财险西藏分公司护航雪域高原春耕好景
- 卜冠今《喜卷常乐城》正在热播 “李宅支柱”谢全佳笑梗不断
- Acronis Welcomes Nexus IT as New #TeamUp Partner for the Utah Jazz
- 移远通信推出全新Wi-Fi 7和蓝牙5.4模组组合,为PC提供巅峰无线连接体验
- “交付力”卷出新高度!2024西安买房还看招商蛇口!
- 曝光成都熠翊发服饰有限公司集合店您给我信任,我还您温馨的童年
- 感仙茶:坚持以质取胜,追求卓越品质
- 跑出激情 美味无限 百胜中国助推春季运动热潮
- 临商银行金雀山支行开展网络安全宣传活动
- 全民质量行动,推动花园牛奶产品服务质量上台阶
- AVEVA推出全新InTouch Unlimited HMI/SCADA功能和定价结构
- Bedford Metals Applies for Exploration Permit for Ubiquity Lake Uranium Project
- 企业所得税九大预警指标
- C.K. McWhorter Endows Sotheby's with the Illustrious McWhorter Family Trust Warrant, Recognizin
- 我国首个远程支持癌症晚期患者公益服务项目及志愿者招募正式启动
- Medisca启用新的药品再包装设施,以满足全球对个性化医疗的需求
- 品冠嗨唱饭撒不停 歌迷激动泪流现场
- 洛微科技新品发布:引领物流与工业自动化产业高端升级新篇章
- 张可盈精彩献唱江苏卫视元宵晚会 飘逸灵动气质绝佳尽显全能实力
- C.K. McWhorter Opens Conversations with Coutts Bank
- 抖音网红低价业务下单24小时,真人播阅读放量粉丝
- 加速量产化节奏!移远通信5G RedCap模组RG255C-CN顺利通过SRRC认证
- Skechers推出高科技仓库,采用Hai Robotics自动化“货到人”系统
- Boomi World 2024用户大会将邀请“最佳教练”Deion Sanders担任主讲嘉宾
- 生态农业引领绿色发展 ———走进大化玲欣家庭农场
推荐
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
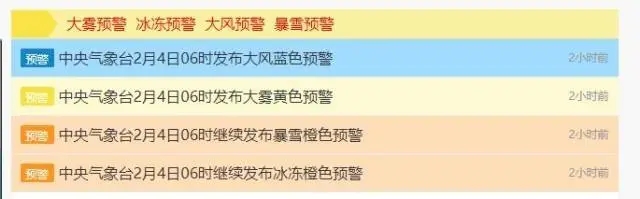 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
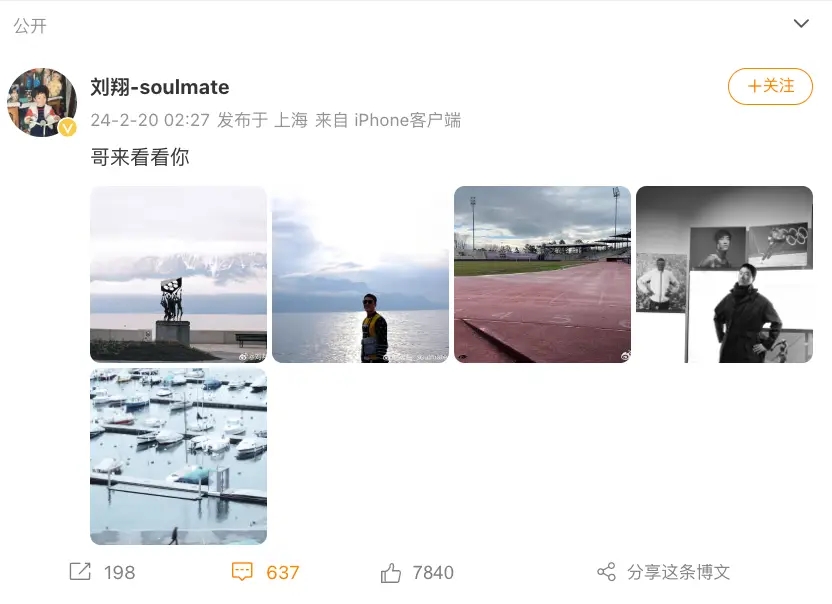 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯

