Skyhawk Therapeutics Announces Positive Topline Results from Parts A and B of its Phase 1 Clinical T
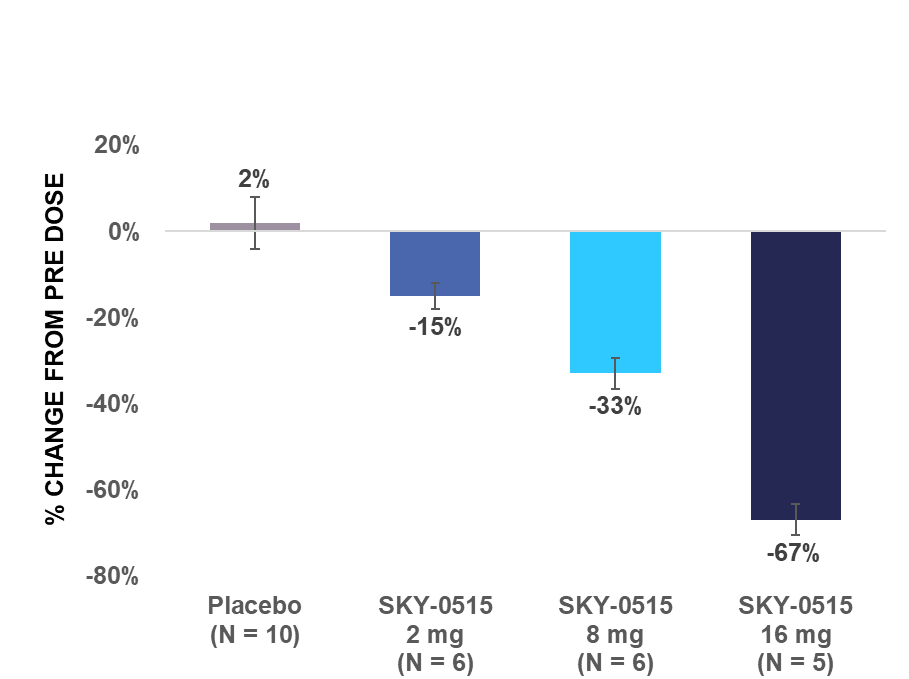
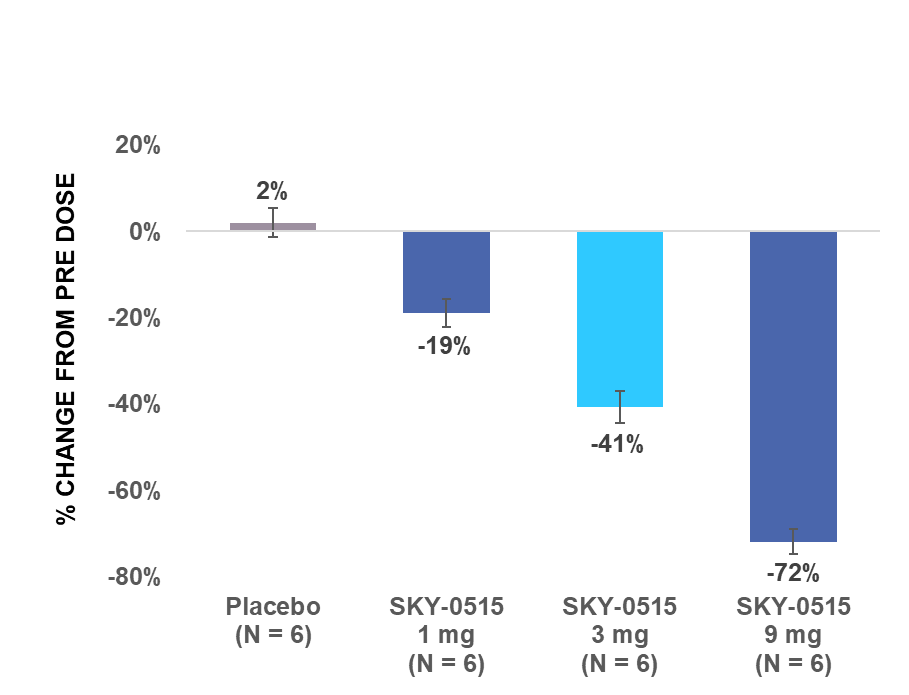
Skyhawk's SKY-0515 demonstrated dose-dependent huntingtin (HTT) mRNA reduction in healthy volunteers, with 72% reduction at the highest dose tested in the multiple ascending dose study
SKY-0515 was generally well tolerated at all doses tested
Given these positive topline results, we anticipate dosing in the patient arm of the study in Q3 2024 and initiation of a Phase 2 study early next year
BOSTON, July 10, 2024 (GLOBE NEWSWIRE) - Skyhawk Therapeutics, Inc., a clinical-stage biotechnology company developing novel small molecule therapies designed to modulate critical RNA targets, today announced positive results from Parts A and B of its Phase 1 clinical trial of SKY-0515, which is being developed as a potential treatment for Huntington's disease (HD). SKY-0515 demonstrated an average HTT mRNA reduction of 72% at a daily oral dose of 9mg and was generally well tolerated at all doses tested.
SKY-0515 is Skyhawk's investigational small molecule RNA splicing modifier developed through the company's novel RNA-splicing platform. SKY-0515 is designed to reduce both HTT protein and PMS1 protein, an additional key driver of somatic CAG repeat expansion and HD pathology.
“Huntingtin-lowering and somatic expansion have been two of the hottest targets in HD research in the past decade. Reducing both HTT and PMS1 could have greater therapeutic benefit than lowering HTT alone,” said Ed Wild, Professor of Neurology at University College London. “Huntington's disease is a rare hereditary neurodegenerative disease affecting over 40,000 patients in the United States. There are no approved treatments that can reverse or slow its course of progression. SKY-0515's HTT reduction has the highest dynamic range I've seen from any therapeutic modality and gives me great hope for SKY-0515's potential to one day help those patients in need.”
“We believe that, with these impressive HTT mRNA lowering results and the drug's predicted suppression of the PMS1 protein, SKY-0515, if approved, could make a meaningful difference in Huntington's patients' lives,” said Douglas V. Faller, M.D., Ph.D., Chief Medical Officer, Skyhawk Therapeutics. “The Safety Review Committee has determined that SKY-0515 has been generally well tolerated at all tested doses with a dose proportional increase in systemic exposure and, given these favorable safety results, approved this study to move into the patient arm. Recruitment has begun, and topline data from this part of the trial are expected to report in Q2 2025.”
“After initiating this Phase 1 clinical trial in late 2023, we're delighted with the speed at which we've conducted this study and thrilled to report such compelling results for SKY-0515,” said Clint Musil, Chief Executive Officer, Skyhawk Therapeutics. “These topline data represent a crucial step forward for SKY-0515 and demonstrate the immense potential of the Skyhawk platform to target indications for which there are no approved disease modifying therapies.”
HTT mRNA levels in the blood from pre-dose are described in the charts below.
| SAD: Maximum reduction in HTT mRNA level in blood from pre-dose within 24 hours after single dose | MAD: Average reduction in HTT mRNA level in blood from pre-dose over 24 hours post dose on day 14 | |
|
|
|
|
| Note: Error bars represent standard error of the mean. | ||
About SKY-0515 Phase 1 Clinical Study
SKY-0515 is currently being evaluated in a Phase 1 clinical trial. The Phase 1 clinical trial is a first-in-human trial designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics, specifically blood biomarker modulation activity, of SKY-0515 in healthy volunteers and individuals with early-stage Huntington's disease (HD). The trial is separated into three parts. Parts A and B evaluated SKY-0515 in healthy volunteers.
Part A was a double-blind placebo-controlled single ascending dose study in healthy adult volunteers. In Part A, five cohorts of participants were dosed with escalating single doses of SKY-0515 ranging from 1mg to 16mg or placebo. Additionally, the influence of food on the pharmacokinetics of SKY-0515 was examined in a dedicated cohort.
Part B was a double-blind placebo-controlled multiple ascending dose study in healthy adult volunteers. In Part B, three cohorts of participants were randomized to receive multiple ascending doses of SKY-0515 ranging from 1mg to 9mg or placebo administered daily from days 1 to 14 (inclusive). Dose levels of SKY-0515, identified in Parts A and B, will be evaluated in Part C.
Part C is a double-blind placebo-controlled parallel design study of two dose levels of SKY-0515 and placebo of individuals with early-stage HD (HD-ISS Stage 1, 2, or mild Stage 3) preceded by an observational period lasting a minimum of 28 days, which aims to evaluate pharmacodynamic parameters such as mutant HTT protein and mRNA. Recruitment for Part C has begun, and topline data are expected in Q2 2025.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company focused on the discovery and development of novel small molecule therapies designed to modulate critical RNA targets and revolutionize patient treatment for some of the world's most intractable diseases. Skyhawk's discovery expertise is rooted in its proprietary drug discovery platform, which assesses, identifies, and tests RNA splicing targets and small molecules across a broad range of therapeutic areas and disease states. Skyhawk has built collaborations with multiple pharma partners that leverage Skyhawk's novel platform across disease areas including neurodegenerative disease, autoimmune disease, and oncology. For more information visit www.skyhawktx.com.
Investor Contact
Anne Marie Fields
Precision AQ (formerly known as Stern Investor Relations)
annemarie.fields@precisionaq.com
332-213-1956
Skyhawk Contact
Maura McCarthy
maura@skyhawktx.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4b26e782-86d2-402d-a4e1-d7892ee21fad
https://www.globenewswire.com/NewsRoom/AttachmentNg/23bc1da1-bf4a-42a8-9d28-1b54d09b7413
- 中信银行大同分行:助力小微尽职责 用心服务获赞许
- 法国娇兰 艺术沙龙 全新香精系列
- 肯德基天使餐厅新拓10城,助残日共创“友爱共融好滋味”
- 【广材试验机】广州+深圳国网卧式拉力试验机报价方案厂家
- 佳兆业美好2023年物业管理服务及社区增值服务收益同比增长7.2% 及13.2%
- UCC3912PWPG4: Revolutionizing Power Management for Next-Generation Electronics | ChipsX
- 申克技术I新一代SmartBalancer4便携式动平衡仪
- 招商云墨 | 高新顶流项目来了,招商书香作品王炸登场!
- 憨萌小狼温暖少女心灵,真事改编治愈电影《薇琪的秘密》欢喜首映独播上线
- EIG旗下MidOcean Energy收购Peru LNG 20%的股份
- Laserfiche人工智能文档摘要:简化内容消费并提高生产力
- 阿斯利康中国IT交付中心: 十年蓄力 科技创新引领智慧医疗新未来
- 连卡佛献礼父亲节 传递真挚心意
- 美酒岛非遗酿艺品鉴馆连锁品牌又获大奖
- 专精特新企业富捷电子再获“高质量发展突出贡献奖”
- 王丹妮复古光影封面大片 氛围独特叙事感十足
- 让爱回家·家和万事兴2024第六届国学晚会在京举办
- Beyond Cyber Protection Leadership: Acronis’ Environmental and Social 2023 ESG Report Revealed
- 全面发力智能化,浪潮海岳发布多项创新成果
- 金湖:网络大V进警营 携手共筑防谣屏障
- 厦门CEIE猫博会 | 瑞派宠物医院许你春日喵喵之约!快来领取签到豪礼~
- 第六届北京大学清明论坛在线上线下举行
- TESAI革新代币玩法,助力全球AI产业
- 无人自助糖水店汕饮糖水在学校附近可以开吗,当然可以!
- ”梅“好”童“行,快乐相随,日客流超10万的这座商业综合体有什么秘诀
- 挚达科技亮相泰国,携手合作伙伴共同推动绿色能源的发展
- 泽信控股集团:深耕匠心品质,坚守重交付良性发展底线
- ExaGrid在2024年存储奖典礼上荣获“年度企业备份硬件供应商”等奖项
- 中国企业家论坛 亚布力时间 布瑞克农业互联网孙彤分享农业数字化转型经验
- 助力打造新质生产力,提高上市公司投资价值—2024中国上市公司论坛在辽宁沈阳成功举办
推荐
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯