Galderma’s Relfydess™ (RelabotulinumtoxinA) Receives Positive Decision for Use in Europe
Ad hoc announcement pursuant to Art. 53 LR
- RelfydessTM (RelabotulinumtoxinA) is the first and only ready-to-use liquid neuromodulator created with PEARLTM Technology, developed and manufactured by Galderma1,2
- This positive decision is based on results from the phase III READY clinical trial program, which showed that RelfydessTM delivered sustained results for six months, combined with an onset of action as early as day one, for both frown lines and crow’s feet3-8
- Galderma is committed to developing and delivering the broadest portfolio in Injectable Aesthetics
- Once national approvals have taken place, RelfydessTM will be the first neuromodulator in Europe to receive initial approval for two indications – frown lines and crow’s feet – at the same time
- RelfydessTM also received marketing authorization from Australia’s Therapeutic Goods Administration in June this year
ZUG, Switzerland -- (BUSINESS WIRE) --
Galderma today announced that it has completed its European decentralized procedure (DCP), resulting in a positive decision for RelfydessTM (RelabotulinumtoxinA – previously referred to as QM1114). RelfydessTM is indicated for the temporary improvement in the appearance of moderate-to-severe glabellar lines (frown lines) at maximum frown and lateral canthal lines (crow’s feet) seen at maximum smile, alone or in combination, in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient.9 Following the successful completion of the DCP, national approvals in the 16 concerned countries are now under finalization. RelfydessTM also received a marketing authorization in Australia earlier this year.
RelfydessTM is developed and manufactured by Galderma. It is the first and only ready-to-use liquid neuromodulator created with PEARL™ Technology that is designed to preserve molecule integrity to deliver a highly active, innovative, complex-free molecule, with up to 39% of patients seeing effects from day one and up to 75% of patients maintaining improvements for six months for frown lines and crow’s feet.3,4,7,8 It is optimized for simple volumetric dosing, without reconstitution, to increase ease of use and help ensure consistent dose/volume.1,10
|
“With RelfydessTM, Galderma is introducing a highly differentiated and innovative neuromodulator, reinforcing our leadership and strong growth in this field, and our commitment to developing and delivering the broadest portfolio in Injectable Aesthetics. As per the decentralized European approach, our teams are now finalizing the approval procedures at the country level, so we’re ready to launch in multiple markets early next year.”
FLEMMING ØRNSKOV, M.D., MPH, CHIEF EXECUTIVE OFFICER GALDERMA
|
This positive decision, and the previous Therapeutic Goods Administration approval in Australia, were based on results from the phase III READY (RElabotulinumtoxin Aesthetic Development StudY) clinical trial program, which enrolled more than 1,900 participants. Results showed:3,4,7,8
- Improvement in both frown lines and crow’s feet versus placebo:
- In READY-1 and READY-2, treatment with RelfydessTM demonstrated a 96.3% none-or-mild responder rate for frown lines and 87.2% for crow’s feet, after one month, vs 4.5% and 11.9% for placebo, respectively.
- Onset of action as soon as day one:
- In READY-1 and READY-2, 39% of patients reported improvements for frown lines and 34% reported improvements for crow’s feet from day one.
- Sustained results for six months:
- In READY-1 and READY-2, up to 75% of patients maintained improvements for six months.
- At month one, up to 96% achieved none-or-mild frown lines and crow’s feet, which was sustained for six months in almost a quarter of patients.
- Patient satisfaction was maintained for six months following treatment.
|
“With the growing need for new innovations in the neuromodulator space, I’m excited that, with RelfydessTM, we have a new treatment that delivers both fast and sustained results in a simple and convenient formulation, so we can achieve the desired outcomes for our patients quickly, effectively, and without compromise.”
DR. SACHIN SHRIDHARANI LEAD INVESTIGATOR OF READY-1 TRIAL PLASTIC SURGEON AND FOUNDER OF LUXURGERY
|
Regulatory applications for Relfydess™ for the treatment of frown lines and crow’s feet will continue to be submitted and assessed by additional authorities globally.
About RelfydessTM (RelabotulinumtoxinA)
Pioneered by Galderma, RelfydessTM is the first and only ready-to-use liquid neuromodulator created with PEARLTM Technology that is designed to preserve molecule integrity.1,2 PEARLTM Technology is designed to deliver a highly active, innovative, complex-free molecule, with up to 39% of patients seeing effects from day one and up to 75% of patients maintaining improvements for six months.1-4,7,8 RelfydessTM is optimized for simple volumetric dosing, without reconstitution, to increase ease-of-use and help ensure consistent dose/volume every time.1,10 It was entirely developed and manufactured by Galderma to expand its neuromodulator portfolio as part of the broadest Injectable Aesthetics portfolio on the market.
About the READY clinical trial program
The READY (RElabotulinumtoxin Aesthetic Development StudY) phase III clinical program is composed of four phase III clinical trials which enrolled more than 1,900 participants.3-6 The READY trials investigated the safety, efficacy, rapidity of onset and/or durability of RelfydessTM for six months on:
- Frown lines (READY-1).3
- Crow’s feet (READY-2).4
- Frown lines and crow’s feet when treated alone or simultaneously (READY-3).5
- Frown lines and crow’s feet when treated alone or simultaneously with up to four repeated injections over 52 weeks (READY-4).6
About Galderma
Galderma (SIX: GALD) is the emerging pure-play dermatology category leader, present in approximately 90 countries. We deliver an innovative, science-based portfolio of premium flagship brands and services that span the full spectrum of the fast-growing dermatology market through Injectable Aesthetics, Dermatological Skincare and Therapeutic Dermatology. Since our foundation in 1981, we have dedicated our focus and passion to the human body’s largest organ – the skin – meeting individual consumer and patient needs with superior outcomes in partnership with healthcare professionals. Because we understand that the skin we are in shapes our lives, we are advancing dermatology for every skin story. For more information: www.galderma.com.
References:
- Sundberg AL and Stahl U. Relabotulinum toxin - a novel, high purity BoNT-A1 in liquid formulation. Presented at: TOXINS 2021; Jan 16-17, 2021; virtual meeting
- Do M, et al. Purification process of a complex-free highly purified botulinum neurotoxin type A1 (BoNT-A1) - relabotulinumtoxinA. Presented at: TOXINS 2022; July 27-30, 2022; New Orleans, LA
- Galderma. Data on file. Clinical Study Report for Protocol 43QM1602: READY-1. Galderma Laboratories; 2021
- Galderma. Data on file. Clinical Study Report for Protocol 43QM1901: READY-2. Galderma Laboratories; 2021
- Galderma. Data on file. Clinical Study Report for Protocol 43QM1902: READY-3. Galderma Laboratories; 2021
- Galderma. Data on file. Clinical Study Report for Protocol 43AM1903: READY-4. Galderma Laboratories; 2021
- Shridharani SM, et al. Efficacy and Safety of RelabotulinumtoxinA, a New Ready-to-Use Liquid Formulation Botulinum Toxin: Results From the READY-1 Double-Blind, Randomized, Placebo-Controlled Phase 3 Trial in Glabellar Lines. Aesthetic Surgery Journal. 2024; sjae131
- Ablon G, et al. Treatment of Lateral Canthal Lines with RelabotulinumtoxinA, an Investigational Liquid Botulinum Toxin: Clinical Efficacy and Safety Results from the READY-2 Phase III Trial. Abstract presented at TOXINS 2024; Jan 17-20, 2024, Berlin
- Relfydess® EU Summary of Product Characteristics
- Persson C, et al. Patient and Investigator Treatment Experience with Ready-to-Use AbobotulinumtoxinA Solution Versus Powder BotulinumtoxinA for Treatment of Glabellar Lines. Abstract presented at TOXINS 2024; Jan 17-20, 2024, Berlin
- 避暑旅游正当时,悟空租车让你清凉一夏
- 解密广西锐翔新型材料公司负氧离子黄晶板整装赋予空间独到的精致感
- 文质彬彬,追求秀雅-李世玉书法作品欣赏
- 车颜知己全自动智能洗车平台,开启深圳市场新篇章!
- 卓翼智能圆满完成深圳市超高层建筑灭火无人机实战应用测试
- 苏一堂紫砂电饭煲,品味健康生活好味道
- 南京汽车救援24小时在线,为您解决汽车故障
- FinalSpark推出首个利用人体神经元进行生物计算的远程研究平台
- 双摄监控无死角,隔空刷掌秒开门 | 乐橙人脸掌静脉带屏视频云锁 LIGHT1-sxp评测
- 易健阳光《冲出重围—变现为王》科技健康5.0峰会在中国佛山隆重举办
- 向上向善,明昇集团积极参与慈善认捐,共筑大爱罗湖
- 孙俪董子健要演姐弟恋?背后真实原因竟如此
- 安顺文化旅游2024(南京)推介会在宁成功举办
- 12.88万起 远程超级VAN上市即交付 全面颠覆传统轻客市场
- 好剧还得看王凯 《大江大河3》收视率一季度第一
- NIQ发布《2024年消费者展望综合报告》:把握全球趋势,洞察战略发展
- SLB OneSubsea获得Equinor北海Troll项目第3阶段第2期合同
- 赋新西安十年,新春买房首选招商蛇口!
- 理大创新科技踏上国际舞台 科研成果首度亮相法国「展翅高飞」展览
- KFSH&RC-Madinah Receives Prestigious 2023 Press Ganey Human Experience Guardian of Excellence Aw
- 税局明确:这8类个人抬头发票不仅可以报销,还可抵扣进项!
- 新疆水博会将举办多场高端论坛探析水利科技创新发展
- Energy Vault 宣布中国天鹰 ( China Tianying ) 成功测试并调试首个 EVx 100 MWh 重力储能系统,并将 Atlas 可再生能源许可协议延长至 15 年
- 电子制造人看过来!NEPCON China 2024四大行业主题日开启探索行业新价值!
- 林宥嘉新专辑《王》LOVE IS KING首唱分享会 分享创作心得 坦露新专辑纪录8年来人生经历
- 下班后独自品味生活 美食和欢笑享受人生
- Brightcove为日本最新流媒体服务——J:COM的“动物观察”提供支持
- 锦江都城荣获双奖,以百年先锋文化铸就中高端酒店行业新典范
- 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
- 「澳門銀河」全新沉浸式展览今日揭幕 荷兰"太阳蛋"首降澳门 纽约"蛋屋"环游而至
推荐
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
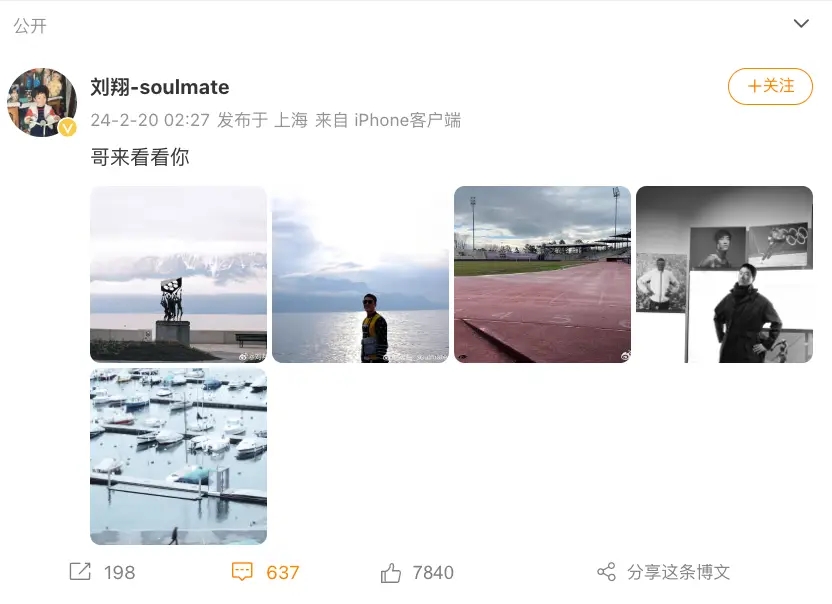 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
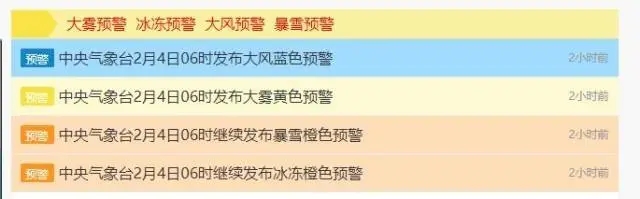 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯

