Galderma Delivers Record Net Sales of 3.259 B USD in the First Nine Months of 2024, Demonstrates Sig
Ad hoc announcement pursuant to Art. 53 LR
ZUG, Switzerland -- (BUSINESS WIRE) --
Galderma Group AG (SIX:GALD), the pure-play dermatology category leader, today announced its sales performance for the first nine months of 2024, reporting record net sales and significant progress across its product categories and key future growth drivers.
- Record net sales: Achieved 3.259 billion USD in net sales during the first nine months of 2024, up +9.2% year-on-year in constant currency1, predominantly driven by volume growth, complemented by favorable mix. Galderma also achieved the highest third quarter net sales in its history
- Broad-based growth: Continued performance across all product categories with sustained double-digit growth in Injectable Aesthetics and Dermatological Skincare, with constant currency year on-year growth of 10.6% for Injectable Aesthetics, 10.6% for Dermatological Skincare, and 2.9% for Therapeutic Dermatology for the first nine months of 2024
- Focused strategic execution: Continued progress against the company’s growth-focused Integrated Dermatology Strategy, advancing all its product categories and future growth drivers through key approvals and bringing to market new products, including Nemluvio® (nemolizumab) for adult patients with prurigo nodularis in the U.S.
- New promising clinical data: Reaffirmed commitment to leading in dermatology with the release of new data, including nemolizumab late-breaking results from ARCADIA and OLYMPIA clinical trial programs in atopic dermatitis and prurigo nodularis
- 2024 full year guidance confirmed and narrowed on net sales: Full year net sales growth expected to be in the range of 8.8-9.5% year-on-year at constant currency (previously expected towards the upper end of the 7-10% growth range at constant currency), and for Core EBITDA margin expected to be in line with 2023 at constant currency
|
“By executing on our unique Integrated Dermatology Strategy, Galderma continues its strong growth trajectory, driven by momentum behind our science-based premium brands. The exciting progress with our late-stage pipeline with key regulatory approvals and differentiated innovations positions Galderma for continued strong growth. Combined with our high-performing commercial execution and increasing penetration in existing and new key markets, this sets the stage for further growth acceleration and value creation.” FLEMMING ØRNSKOV, M.D., MPH
|
Delivering strong commercial performance
In the first nine months of 2024, Galderma achieved record net sales of 3.259 billion USD, representing year-on-year net sales growth of 9.2% at constant currency, primarily driven by volume growth, complemented by favorable mix.
Net sales growth was widespread across product categories and geographies. All product categories grew, with notable sustained double-digit growth in Injectable Aesthetics and Dermatological Skincare.
Growth is increasingly driven by Galderma’s larger reporting geography, International, representing 59% of Group sales at the end of the nine-month period and where Galderma remains underpenetrated. International markets maintained double-digit growth momentum fueled by strong performance across major markets, with, notably, China continuing its strong trajectory across both Injectable Aesthetics and Dermatological Skincare. The U.S. grew single digits during the first nine months of the year despite a softer market environment, impacted by the continued softness in Fillers and more cautious consumer consumption in the Dermatological Skincare market segment.
Injectable Aesthetics
Injectable Aesthetics net sales for the first nine months of the year were 1,698 million USD, with year-on-year growth of 10.6% at constant currency.
Both Injectable Aesthetics sub-categories achieved double-digit growth. Neuromodulators net sales were 926 million USD, up 10.4% year-on-year at constant currency, while Fillers and Biostimulators net sales were 772 million USD, up 10.9% year-on-year at constant currency.
Growth for the product category was supported by strong performance in both International markets and the U.S., noting that International continued its double-digit growth trajectory. Neuromodulators experienced particularly strong demand in Europe and Latin America, while Fillers was impacted with market softness despite some markets with strong growth such as in Asia Pacific. Biostimulators continued its strong, broad-based growth trajectory supported by the successful Sculptra® launch in Thailand earlier this year and strong demand across key markets, with notable outperformance in the Middle East.
Dermatological Skincare
Dermatological Skincare net sales for the first nine months of the year were 990 million USD, growing by 10.6% year-on-year at a constant currency basis.
Growth was driven by its two flagship brands, Cetaphil® and Alastin®. Cetaphil growth was particularly strong in International markets more than offsetting lower consumer consumption in the U.S., while Alastin continued its strong U.S. growth across channels.
Therapeutic Dermatology
Therapeutic Dermatology net sales for the first nine months of the year were 571 million USD, with year-on-year growth of 2.9% on a constant currency basis.
Growth was mainly driven by continued momentum in International markets, which offset the anticipated decline in the U.S., impacted by ongoing genericization. In addition, Galderma recorded its first sales for Nemluvio® (nemolizumab), which was made available in August after the U.S. Food and Drug Administration (FDA) approval with priority review for adults with prurigo nodularis. This represents an important milestone, which sets Galderma up for strong growth in Therapeutic Dermatology and in the U.S.
Q3 highlights: making significant headway across all product categories and future growth drivers
Galderma continued to make strong progress during the third quarter of 2024 across its product categories, securing key regulatory approvals and launching new products expected to further fuel its growth trajectory.
In Injectable Aesthetics, Galderma completed its European decentralized procedure (DCP), resulting in a positive decision for Relfydess™ (RelabotulinumtoxinA, previously referred to as QM1114). Relfydess is indicated for the temporary improvement in the appearance of moderate-to-severe glabellar lines (frown lines) at maximum frown and lateral canthal lines (crow’s feet) seen at maximum smile, alone or in combination, in adult patients under 65 years, when the severity of these lines has an important psychological impact on the patient. Following the successful completion of the DCP, national approvals in the 16 concerned countries are now under finalization with approvals already received in five European markets as well as in Australia. As a further update to neuromodulators, the arbitration proceeding initiated by Galderma against Ipsen at the International Chamber of Commerce (ICC) related to the territorial scope of the Azzalure® and Dysport® commercial partnership under the 2007 European development and distribution agreement has now been completed. The Tribunal of the ICC issued a final award in October 2024, reaffirming the scope of Galderma’s exclusive distribution rights with respect to Azzalure and Dysport. The award confirmed Galderma’s rights to commercialize Azzalure and Dysport in certain additional countries, including in Eastern Europe and Central Asia, that had been in dispute between the parties but dismissed Galderma’s claim for monetary compensation related to the timing of the transfer of such rights. Galderma and Ipsen continue to work in close partnership to grow Azzalure and Dysport sales in the territories of their commercial alliance.
In the biostimulator sub-category of Injectable Aesthetics, Galderma received approval for Sculptra in China, one of the world’s fastest growing aesthetics markets. As the first and original biostimulator, with a unique PLLA-SCA™ formulation that activates the skin’s natural power to revitalize collagen production for a more youthful appearance, Sculptra further completes our portfolio offering in China.
In Dermatological Skincare, Galderma continued to invest behind Cetaphil’s growth, with a re-energized brand, focused retailer execution, and a digital-first activation. This included upgraded placement in Walmart in the U.S. with a multi-channel activation. Galderma also introduced in the U.S. the new Cetaphil Gentle Exfoliating line to provide gentle chemical exfoliation that is suitable for daily use, even on the most sensitive skin. The launch campaign of this new Cetaphil line, developed with dermatologists, featured Gen-Z influencer Katie Fang, as part of its strengthened advocacy-driven strategy. In the peri-procedure skincare category, Alastin continued its focused execution in the U.S., along with further expanding its international footprint with the launch of Alastin in Brazil and Colombia in September.
In Therapeutic Dermatology, the U.S. FDA approved Nemluvio (nemolizumab) as a pre-filled pen for subcutaneous injection for the treatment of adults with prurigo nodularis. Nemluvio was granted Breakthrough Therapy Designation in December 2019 and Priority Review in February 2024 by the U.S. FDA, a status reserved for medicines with the potential to significantly improve the treatment of serious conditions. Following approval, Nemluvio was administered in the first patient within two days and has since been prescribed by approximately 1,100 healthcare professionals for this rare condition. Nemluvio is also under review by the U.S. FDA for the treatment of adults with moderate-to-severe atopic dermatitis, with a decision anticipated later this year. Marketing authorization applications for Nemluvio in both prurigo nodularis and atopic dermatitis are currently under review by multiple regulatory authorities, including the European Medicines Agency and Health Canada, as well as in Australia, Singapore, Switzerland, and the United Kingdom, via the Access Consortium framework.
Further differentiating the broadest dermatology portfolio with breakthrough science
Galderma reaffirmed its leadership in dermatology with a strong presence at key industry events, showcasing its innovative, science-based portfolio that spans the full spectrum of the fast-growing dermatology market.
At the 33rd European Academy of Dermatology and Venereology (EADV) Congress, Galderma presented 30 abstracts featuring data on prurigo nodularis, atopic dermatitis, sensitive skin and acne. This included late-breaking data from the ARCADIA and OLYMPIA clinical trial programs investigating nemolizumab in atopic dermatitis and prurigo nodularis. These new findings build on previously published results from the robust phase III ARCADIA and OLYMPIA clinical trial programs and demonstrate nemolizumab’s long-term efficacy and safety in atopic dermatitis and durability in prurigo nodularis.
Full results from nemolizumab’s phase III ARCADIA 1 and 2 clinical trials in atopic dermatitis were published in The Lancet. These trials evaluated the efficacy and safety of nemolizumab in combination with background topical corticosteroids (TCS), with or without topical calcineurin inhibitors (TCI), versus placebo in combination with TCS, with or without TCI, in adolescent and adult patients with moderate-to-severe atopic dermatitis. The trials met their co-primary and all key secondary endpoints, showing that nemolizumab significantly improved skin lesions, itch and sleep disturbance by Week 16 when compared to placebo, with significant itch relief observed as early as Week 1.
Galderma also announced a memorandum of understanding with L’Oréal in August 2024 to work towards a new research and development collaboration. The partnership being explored, with discussions ongoing and progressing well, would focus on complementary research projects aimed at developing advanced, future-proof technologies with direct applications in dermatology. At the same time, Galderma welcomed L’Oréal as a new shareholder with its acquisition of a 10% stake in Galderma, following an agreement between Sunshine SwissCo AG (a consortium led by EQT), Abu Dhabi Investment Authority (ADIA) and Auba Investment Pte. Ltd.
Strengthening Galderma’s financial profile
Underscoring its continued strong performance and momentum since its Initial Public Offering (IPO) in March 2024, Galderma was added to the Swiss Market Index Mid (SMIM), which lists the 30 next most liquid and largest equity instruments traded at SIX that are not in SMI, as well as to the FTSE Global Equity Index Series (GEIS), both effective from September 23, 2024. These listings further highlight Galderma’s growing prominence in the market, adding to its earlier inclusion in the MSCI World Index and STOXX Europe 600 Index.
In addition, Galderma successfully issued an inaugural bond with a total amount of CHF 500 million through a dual tranche offering, the net proceeds of which were used for the partial refinancing of its existing Bank Term Loan issued at IPO and general purposes. The issuance was leverage-neutral and doesn’t affect the leverage and interest cash expense for the second half of 2024, as communicated on July 25, 2024.
Confirming full year guidance
In light of the strong performance achieved in the first nine months of the year and expected for the last quarter of the year, Galderma is confirming and narrowing its net sales full year guidance to a net sales growth of 8.8-9.5% year-on-year at constant currency, which was previously raised towards the upper end of the 7-10% growth range at constant currency. Full year Core EBITDA margin is confirmed to be in line with 2023 at constant currency.
Webcast details
Galderma will host its financial results call today at 14:00 CEST to discuss nine-month results and respond to questions from financial analysts. Investors and the public may access the webcast by registering on the Galderma Investor Relations website at https://investors.galderma.com/events-presentations.
- 小柯首创地标性旅游大秀《艺术区》盛放751北京戏剧嘉年华
- 位元堂药业捐赠港币一百万元予广州中医药大学 成立「位元堂奖学金」推动中医药行业发展
- 甜梦常伴,Simmons®席梦思为中国SOS儿童村送去母亲节的温暖
- 瑞派公益 | 瑞派宠物医院助力CRC狂犬病防治项目,开展春季暖心公益活动
- Rimini Street Japan Celebrates 10 Years of Extraordinary Client Service and Regional Success
- 最受酒店欢迎投影仪:影宿智联投影亮相2024上海国际酒店展
- 照亮“一带一路” 三雄极光在延吉迈出新步伐!
- 律师管理系统优选Alpha,专为律师事务所打造的律师办公软件
- 《致命游戏》谁最火?徐冬冬有效助力爆剧收视热度高涨
- 锦勇,气密型化学防护服,适用化工厂、实验室、清洁剂生产车间等
- 蔺建军汽奶获【AIFF金鹮奖】亚洲时尚健康饮料品牌奖
- 联拓生物宣布自愿从纳斯达克退市
- 第七届“绽放杯”5G应用征集大赛 5G应用融合技术专题赛圆满收官
- FPT Software Recognized as a Major Player in the IDC MarketScape: Asia/Pacific Managed Security Serv
- 支部联学聚合力 党建共建促发展
- 贴身衣服品牌有棵树官宣成毅为全球品牌代言人,彼此成就共赴美好
- 旧衣物回收市场如何助力实现双碳目标
- OSL 获任命为嘉实国际资产现货虚拟货币交易所买卖基金的首家虚拟资产交易平台及托管方
- 院线电影《天空依旧》演员谭诗雨演绎家庭亲情
- MC08XS6421EK: Power Management Solution for Next-Generation Electronics | ChipsX
- 携手知“食”达人,黄浦两大商圈举办舌尖上的创意盛宴
- 《树下有片红房子》开机 唤醒90后青葱岁月集体记忆
- MultiBank Group被FX Empire评为2024年最佳金牌经纪商
- 梁心颐《百分百歌手》开播 《下雨天》大合唱满满感动
- Nasdaq Expands Digital Bank FinTech Presence in Latin America
- 2024全球超模大赛(北京|山东|内蒙三城联动)顺利举办
- “以智慧 悦生活”——悦宠生活携手卡奥斯创智物联在宠物设备智造生态领域达成战略合作
- 中南集团与固安县政府正式签约 ,共同书写地方经济繁荣的新篇章
- 刘万鸣 | 相由心生
- 张家辉二度被邀韩国富川影展 《赎梦》入围奇幻Mad MaxX单元
推荐
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
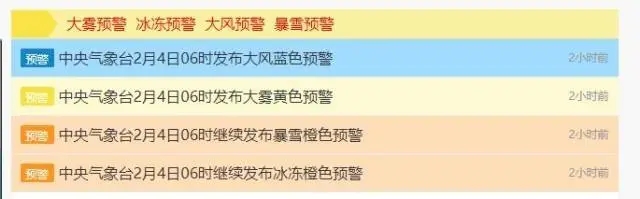 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯

