Zymeworks Presents New Preclinical Data on Antibody-Drug Conjugate Programs at EORTC-NCI-AACR Confer

- Presentations highlight key preclinical data that support investigational new drug application (IND) submissions for ZW220 in 1H and ZW251 in 2H in 2025
VANCOUVER, British Columbia, Oct. 25, 2024 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a clinical-stage biotechnology company developing a diverse pipeline of novel, multifunctional biotherapeutics to improve the standard of care for difficult-to-treat diseases, today announced new preclinical data for Zymeworks’ antibody-drug conjugate (ADC) candidates ZW220 and ZW251 in presentations at the European Organisation for Research and Treatment of Cancer-National Cancer Institute-American Association for Cancer Research (EORTC-NCI-AACR) Conference taking place in Barcelona on October 23-25, 2024.
"We are thrilled to present preclinical data for ZW220 and ZW251 at the EORTC-NCI-AACR Conference," said Paul Moore, Ph.D., Chief Scientific Officer at Zymeworks. "These innovatively designed molecules, incorporating our proprietary payload, ZD06519, show significant potential to address critical unmet needs in oncology. The preclinical data presented this week supports our belief that these programs could advance treatment options beyond current standards of care, offering renewed hope for patients battling challenging cancers where therapeutic progress has been limited."
Oral Presentation Details
An oral presentation titled “ZW220, a NaPi2b-directed topoisomerase I inhibitor Antibody-Drug Conjugate, demonstrates compelling preclinical activity in NSCLC, ovarian and uterine cancer models, with a favorable toxicology profile in non-human primate” highlights preclinical data that continue to support an IND submission in the first half of 2025.
Results demonstrate that ZW220 has the potential for improvement over previous NaPi2b ADCs and on the basis of efficacy, tolerability and payload mechanism. ZW220 features a novel, moderate potency TOPO1i payload with strong bystander activity, beneficial for tumors with low and heterogeneous NaPi2b expression. We believe it offers a differentiated safety profile compared to other ADCs currently in the clinic, demonstrating high tolerability in animal studies with MTD ≥90 mg/kg in non-human primates (NHP) and ≥200 mg/kg in rats, suggesting potential for high doses in humans. The low drug-antibody ratio (DAR) and moderate stability of the antibody-linker provide a good balance of stability, tolerability, and anti-tumor activity, while potentially minimizing antibody-driven toxicities. ZW220's strong internalizing antibody enables efficient cellular trafficking and tissue penetration, potentially improving anti-tumor activity even at lower NaPi2b levels.
Poster Presentation Details
A poster titled “ZW251, a novel glypican-3-targeting antibody-drug conjugate bearing a topoisomerase I inhibitor payload, demonstrates compelling preclinical activity in hepatocellular carcinoma models” highlights preclinical data that continue to support an IND submission in the second half of 2025.
Results demonstrate that ZW251 shows promise as a new treatment option for patients, potentially improving upon the current standard of care (SOC). Demonstrating strong anti-tumor activity across a wide range of hepatocellular carcinoma (HCC) models, including those with lower and heterogenous GPC3 expression, ZW251 was designed with a DAR of four, striking a balance between tolerability and broad anti-tumor effectiveness. In NHP studies, ZW251 displayed significant tolerability at doses up to 120 mg/kg. As an ADC, ZW251 offers flexibility in treatment strategies, potentially serving as either a standalone therapy or in combination with existing SOC treatments.
About ZW220
ZW220 is an ADC that targets NaPi2b-expressing NSCLC and ovarian cancers, and is built, like ZW191, using our proprietary TOPO1i- payload technology. The NaPi2b-targeting monospecific antibody incorporated in ZW220 was developed in-house and selected based on a favorable binding profile and enhanced internalization properties to enable targeting of both high- and low- NaPi2b-expressing tumors. The antibody in ZW220 is Fc-silenced, containing mutations in its Fc region which abolish FcγR (Fc gamma receptor) binding in normal cells, with the goal of minimizing potential off-target toxicities. A DAR of four and a moderate stability antibody-linker were selected to balance tolerability and efficacy. NaPi2b is reported to be expressed in approximately 96% of ovarian serous adenocarcinoma and 87% of non-small cell lung cancer (NSCLC) adenocarcinoma1, and ZW220 has demonstrated anti-tumor activity in patient-derived xenograft models and robust growth inhibition in 3D spheroid models of these cancers. The bystander effect of the TOPO1i payload may help address NaPi2b heterogeneity across these cancers. ZW220 is well-tolerated in non-human primates (NHP) and rats, reaching maximum tolerated doses (MTDs) of ≥ 90 mg/kg and ≥ 200 mg/kg, respectively. We expect to submit an investigational new drug application (IND) and non-U.S. applications for ZW220 in the first half of 2025.
About ZW251
ZW251, a potential first-in-class ADC molecule designed for the treatment of glypican 3 (GPC3)-expressing HCC, incorporates the same Zymeworks proprietary bystander-active TOPO1i payload utilized in ZW191 (anti-FRα) and ZW220 (anti-NaPi2b). A DAR of four and a moderate stability antibody-linker were selected to balance tolerability and efficacy. In preclinical studies, anti-tumor activity for ZW251 was observed in multiple patient-derived xenograft models of HCC reflecting a range of GPC3 over-expression. GPC3, a glycosylphosphatidylinositol (GPI)-anchored cell surface oncofetal antigen, is over-expressed in most HCC patients (>75%2), and displays minimal normal adult tissue expression, making it an appealing ADC target. In NHP studies, ZW251 displayed significant tolerability at doses up to 120 mg/kg, suggesting the possibility of high initial dosing in human trials. We are encouraged by published research demonstrating the potential of GPC3-targeting antibody in HCC patients as evidenced by tumor localization of iodine radiolabeled condrituzumab, a prior clinical-stage anti-GPC3 mAb, and believe that ADC-based targeting of GPC3 could enable a novel and effective approach to treatment of HCC. We expect to submit an IND and non-U.S. applications for ZW251 in the second half of 2025.
About Zymeworks Inc.
Zymeworks is a global clinical-stage biotechnology company committed to the discovery, development, and commercialization of novel, multifunctional biotherapeutics. Zymeworks’ mission is to make a meaningful difference in the lives of people impacted by difficult-to-treat cancers and other diseases. The Company’s complementary therapeutic platforms and fully integrated drug development engine provide the flexibility and compatibility to precisely engineer and develop highly differentiated antibody-based therapeutic candidates. Zymeworks engineered and developed zanidatamab, a HER2-targeted bispecific antibody using the Company’s proprietary Azymetric™ technology. Zymeworks has entered into separate agreements with BeiGene, Ltd. (BeiGene) and Jazz Pharmaceuticals Ireland Limited (Jazz), granting each exclusive rights to develop and commercialize zanidatamab in different territories. Zanidatamab is currently being evaluated in multiple global clinical trials as a potential best-in-class treatment for patients with HER2-expressing cancers. A Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) seeking accelerated approval for zanidatamab as a treatment for previously-treated, unresectable, locally advanced, or metastatic HER2-positive biliary tract cancer (BTC) has been accepted and granted Priority Review. A BLA has also been accepted for review by the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) in China. If approved, zanidatamab would be the first HER2-targeted treatment specifically approved for BTC in the U.S. and China. Zymeworks is rapidly advancing a robust pipeline of wholly-owned product candidates, leveraging its expertise in both antibody-drug conjugates and multispecific antibody therapeutics targeting novel pathways in areas of significant unmet medical need. Phase 1 studies for ZW171 and ZW191 are now actively recruiting. In addition to Zymeworks’ pipeline, its therapeutic platforms have been further leveraged through strategic partnerships with global biopharmaceutical companies. For information about Zymeworks, visit www.zymeworks.com and follow @ZymeworksInc on X.
Forward Looking Statements
This press release includes “forward-looking statements” or information within the meaning of the applicable securities legislation, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements in this press release include, but are not limited to, statements that relate to anticipated preclinical data presentations; the timing and status of ongoing and future studies and the release of data; expectations regarding future regulatory filings and approvals and the timing thereof; the timing of and results of interactions with regulators; Zymeworks’ preclinical pipeline; the anticipated benefits of the collaboration agreements with Jazz and BeiGene; the commercial potential of technology platforms and product candidates; Zymeworks’ clinical development of its product candidates and enrollment in its clinical trials; the potential addressable market of zanidatamab; potential safety profile and therapeutic effects of zanidatamab and Zymeworks’ other product candidates; the ability to advance product candidates into later stages of development; and other information that is not historical information. When used herein, words such as “plan”, “believe”, “expect”, “may”, “anticipate”, “potential”, “will”, “continues”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks’ current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation: clinical trials and any future clinical trials may not demonstrate safety and efficacy of any of Zymeworks’ or its collaborators’ product candidates; any of Zymeworks’ or its partners’ product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; regulatory agencies may impose additional requirements or delay the initiation of clinical trials; the impact of pandemics and other health crises on Zymeworks’ business, research and clinical development plans and timelines and results of operations, including impact on its clinical trial sites, collaborators, and contractors who act for or on Zymeworks’ behalf; the impact of new or changing laws and regulations; market conditions; inability to maintain or enter into new partnerships or strategic collaborations; and the factors described under “Risk Factors” in Zymeworks’ quarterly and annual reports filed with the Securities and Exchange Commission (copies of which may be obtained at www.sec.gov and www.sedar.com).
Although Zymeworks believes that such forward-looking statements are reasonable, there can be no assurance they will prove to be correct. Investors should not place undue reliance on forward-looking statements. The above assumptions, risks and uncertainties are not exhaustive. Forward-looking statements are made as of the date hereof and, except as may be required by law, Zymeworks undertakes no obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events.
Contacts:
Investor Inquiries:
Shrinal Inamdar
Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media Inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com
1Lin et al. 2015. Clin Cancer Res
2Wang HL et al., Arch Pathol Lab Med 2008
- 西安:千年古都,魅力新篇
- Kirin Holdings开始要约收购FANCL以使其成为全资子公司
- 参领生态,智创未来 参天亮相第二十八次全国眼科学术大会
- 赋能新疆大坚果,疆果果打造坚果零食品牌价值
- 沈阳市第六人民医院:构建智能化影像诊断体系,让医疗服务更精准、更高效
- 超大变温魔术舱,澳柯玛537冰箱领鲜来袭
- Information on the total number of voting rights and shares
- Canva凭借在Canva Create首次亮相的全新强大职场产品开启企业应用时代
- 国货品牌之光,不忘初心,于楠,凯萨蒂持续深耕品牌实力!
- 直流dc500v550v600v转变ac220V正弦波逆变器
- 探索新安全、新智慧、新绿色的未来民航——inter airport China 2024机场展共话前沿科技与创新实践
- 吕志和博士获委任为复旦大学资深校董 以董事会永久成员身份传承教育使命
- 充分发挥保险“减震器”与“稳定器”功能,中国人寿让保险“大有作为”
- 淘宝·山西年货节:共赴特色文化盛宴
- 世贸通美国EB5投资移民:2024年11月美国移民签证新公告出炉!
- View韩国地包天手术面诊指南 | 应该沟通些什么?
- 蔡徐坤录音室日常大公开 发布创作花絮照引众人期待
- 临商银行成都路支行举办实物贵金属业务营销培训
- 电视剧《流水迢迢》开播 陈依莎一人千面展现小角色光芒
- 2024IOTE物联网展:跟随利尔达洞见未来
- Moody’s宣布参与守护者计划(Project Guardian),探索资产代币化
- 足力健老人鞋:再次成为中国连锁经营协会会员单位
- Moove获得1亿美元B轮融资
- 小柯全新音乐剧《深处》用真心再创奇迹,高朋满座,好评如潮!
- 清隽素雅·芳韵连绵——国画名家陆瑜百家媒体聚焦报道
- Anaqua and AnyGen AI Form Strategic Partnership to Deliver AI Solutions to the IP Market
- 民间土八路中医——陈彩声
- Aster DM Healthcare旗下的九家医院入选《新闻周刊》“2024年全球最佳医院”榜单
- akeda宣布新的董事任命
- 中信银行太原分行:国际业务线上化产品全面落地 助力企业高质量发展
推荐
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
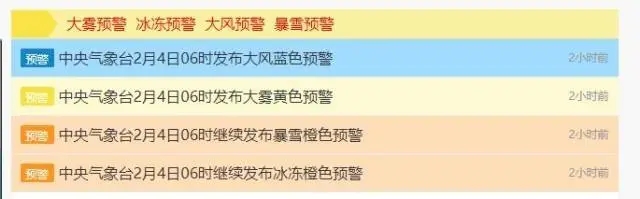 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯

