Zymeworks Announces Achievement of $14 Million Milestone from GSK Collaboration

VANCOUVER, British Columbia, Feb. 26, 2025 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME) a clinical-stage biotechnology company developing a diverse pipeline of novel, multifunctional biotherapeutics to improve the standard of care for difficult-to-treat diseases, including cancer, inflammation, and autoimmune disease, today announced achievement of a $14 million cash research milestone from GSK associated with a clinical milestone.
About the Zymeworks and GSK Collaboration
In April 2016, we entered into a platform technology transfer and license agreement with GSK to research, develop and commercialize up to six bispecific antibodies generated using our Azymetric™ platform. Under the terms of this agreement, we granted GSK a worldwide, royalty-bearing antibody sequence pair-specific exclusive license to research, develop and commercialize licensed products. In May 2019, this agreement was expanded to provide GSK access to Zymeworks’ unique heavy-light chain pairing technology under the Azymetric™ platform.
Under the terms of this expanded collaboration agreement with GSK, we previously received an upfront technology access fee payment, and we remain eligible to receive research, development, and commercial milestone payments of up to $1.1 billion. In addition, we are eligible to receive tiered royalties on worldwide sales.
About the Azymetric™ Platform
Azymetric™ is a heterodimeric antibody technology that gives the ability to engineer, screen, and effectively choose the optimal geometry and valency for our targeted treatments. These customized therapeutic antibodies are engineered to simultaneously bind to multiple distinct locations on a target or to multiple targets, resulting in unique mechanisms of action not accessible through typical monospecific antibodies. Azymetric antibodies can block multiple signaling pathways, recruit immune cells to tumors, enhance receptor clustering and internalization, and increase tumor-specific targeting. Zymeworks’ other technologies can combine with Azymetric to engineer the antibody backbone of a bispecific antibody-drug conjugate or the base of a multispecific therapeutic, to potentially overcome known therapeutic barriers and help design potential best-in-class bispecifics and trispecifics.
Clinical validation of the Azymetric platform is demonstrated by the accelerated approval of Ziihera® (zanidatamab-hrii), a treatment for advanced HER2-positive biliary tract cancer in adults who have received prior therapy, our partner Jazz Pharmaceuticals received from the U.S. Food and Drug Administration in 2024.
About Zymeworks Inc.
Zymeworks is a global clinical-stage biotechnology company committed to the discovery, development, and commercialization of novel, multifunctional biotherapeutics. Zymeworks’ mission is to make a meaningful difference in the lives of people impacted by difficult-to-treat conditions such as cancer, inflammation, and autoimmune disease. The Company’s complementary therapeutic platforms and fully integrated drug development engine provide the flexibility and compatibility to precisely engineer and develop highly differentiated antibody-based therapeutic candidates. Zymeworks engineered and developed zanidatamab, a HER2-targeted bispecific antibody using the Company’s proprietary Azymetric™ technology. Zymeworks has entered into separate agreements with BeiGene, Ltd. (BeiGene) and Jazz Pharmaceuticals Ireland Limited (Jazz Pharmaceuticals), granting each exclusive rights to develop and commercialize zanidatamab in different territories. The U.S. FDA granted accelerated approval of Ziihera® (zanidatamab-hrii) 50mg/mL for injection for intravenous use for the treatment of adults with previously-treated, unresectable or metastatic HER2-positive (IHC 3+) second-line biliary tract cancer (BTC). Ziihera® is the first and only dual HER2-targeted bispecific antibody approved for HER2-positive BTC in the U.S. Zanidatamab is currently under regulatory review in the EU and China for second-line BTC and is being evaluated in multiple global clinical trials as a potential best-in-class treatment for patients with multiple HER2-expressing cancers. Zymeworks is rapidly advancing a robust pipeline of wholly-owned product candidates, leveraging its expertise in both antibody-drug conjugates and multispecific antibody therapeutics targeting novel pathways in areas of significant unmet medical need. Phase 1 studies for ZW171 and ZW191 are now actively recruiting with investigational new drug applications for ZW220 and ZW251 planned for 2025. In addition to Zymeworks’ pipeline, its therapeutic platforms have been further leveraged through strategic partnerships with global biopharmaceutical companies. For information about Zymeworks, visit www.zymeworks.com and follow @ZymeworksInc on X.
Cautionary Note Regarding Forward-Looking Statements
This press release includes “forward-looking statements” or information within the meaning of the applicable securities legislation, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements in this press release include, but are not limited to, statements that relate to the anticipated benefits of the collaboration agreement with GSK; ongoing clinical studies and regulatory reviews; Zymeworks’ ability to receive additional payments pursuant to its collaboration agreements, including any future milestone payments and royalties; the potential addressable market of zanidatamab; the timing of and results of interactions with regulators; Zymeworks’ clinical development of its product candidates and enrollment in its clinical trials; the timing and status of ongoing and future studies and the related data; expectations regarding future regulatory filings and approvals and the timing thereof; potential safety profile and therapeutic effects of zanidatamab and Zymeworks’ other product candidates; the commercial potential of technology platforms and product candidates; Zymeworks’ ability to satisfy potential regulatory and commercial milestones with existing and future partners; anticipated continued receipt of revenue from existing and future partners; Zymeworks’ ability to execute new collaborations and partnerships and other information that is not historical information. When used herein, words such as “plan”, “believe”, “expect”, “may”, “anticipate”, “potential”, “will”, “continues”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks’ current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation: any of Zymeworks’ or its partners’ product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; Zymeworks may not achieve milestones or receive additional payments under its collaborations; regulatory agencies may impose additional requirements or delay the initiation of clinical trials; the impact of new or changing laws and regulations; market conditions; the impact of pandemics and other health crises on Zymeworks’ business, research and clinical development plans and timelines and results of operations, including impact on its clinical trial sites, collaborators, and contractors who act for or on Zymeworks’ behalf; zanidatamab may not be successfully commercialized; clinical trials and any future clinical trials may not demonstrate safety and efficacy of any of Zymeworks’ or its collaborators’ product candidates; and Zymeworks may be unable to maintain or enter into new partnerships or strategic collaborations.
Although Zymeworks believes that such forward-looking statements are reasonable, there can be no assurance they will prove to be correct. Investors should not place undue reliance on forward-looking statements. The above assumptions, risks and uncertainties are not exhaustive. Forward-looking statements are made as of the date hereof and, except as may be required by law, Zymeworks undertakes no obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events.
Contacts:
Investor Inquiries:
Shrinal Inamdar
Senior Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media Inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com
- 三证齐全!吉因加荣获CLIA认证,国际质量标准再升级
- 倍欣使用的用法用量及注意事项
- 3000亩田园养成,颜值品质出圈,度假小高层10#热势加推!
- 南京移动携手警方重拳出击,捣毁机票退改签诈骗团伙
- 传递更深层次美学体验与情感共鸣 大王椰“大师系列”新品上市
- 2024“翠岗红旗杯”宁都红色马拉松赛落下帷幕社会各界反响热烈,好评如潮
- Pharmanovia与Lindis Biotech签订生物新疗法引入许可协议,将用于治疗罕见疾病恶性腹水的卡妥索单抗商业化
- 甘肃温湿度记录标签的创新:提高数据准确性和追溯能力的新技术
- 妻子能否追回丈夫赠给情人钱款?
- 殷桃剧情真实到太勾心 网友直呼怪不得她是大满贯
- KnowBe4将8月6日定为全国社会工程日
- 美赞臣持续推动环保实践 再次启动「罐」盖新生奶粉罐收集计划
- 图格医疗完成近2亿元B轮融资 自主原研 引领革新
- 月销连破8千 ,北汽极狐5系双子星启动3.1888万限时钜惠,打响新一轮价值战!
- 展现智能与传统的激情碰撞
- 健康鲜呼吸,澳柯玛2024空调新品正式发布
- 中成药传承创新——山东汉方创造新辉煌
- 王骁领衔主演剧集《凡人歌》收官 描摹生活底色唱响凡人心声
- WS在创意的海洋中航行:WhatsApp营销工具助您开创业务的全新篇章
- 2024年成都熠翊发服饰有限公司童装集合店成行业中的宠儿
- 酷家乐母公司群核科技拟来港IPO 冲击「全球空间智能第一股」
- 陵水环岛旅游公路:和美乡村新景 生态文旅并进
- 《中国近现代名家画集·马国强》(大红袍)首发式暨新书捐赠仪式在郑州举办
- 江西省台办组织11家台企参加东莞台博会
- 热烈庆祝葫芦嘴品牌纪录片央视频热播中
- 泰雷兹拓展CipherTrust数据安全平台即服务产品系列
- 中医药杰出贡献人物——朱怀安
- ATNi Launches 5th Global Access to Nutrition Index as Industry and Policy Makers Grapple with Food P
- 国际市场“开门红”!翊新诊断携新品闪耀2024ADLM芝加哥
- Vista marks 20 years of success with strong 2023 performance
推荐
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
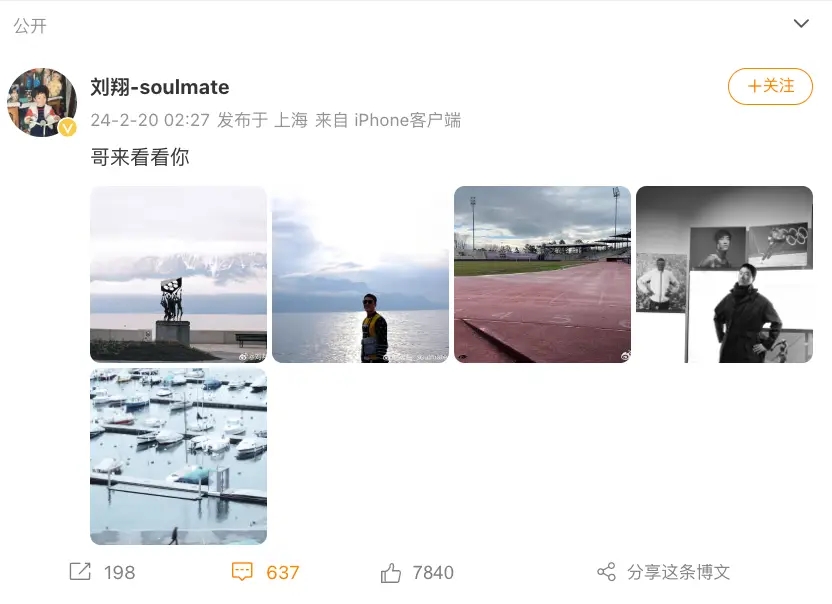 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
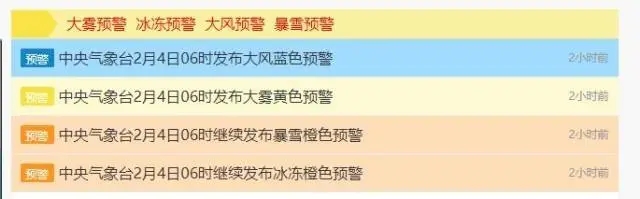 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯

