Novotech Publishes Research Report on Acute Myeloid Leukaemia Clinical Trial Landscape for Clinical
Acute Myeloid Leukemia (AML)
Global Clinical Trial Landscape (2024)
BOSTON, April 10, 2024 (GLOBE NEWSWIRE) -- Novotech, the global full-service clinical Contract Research Organization (CRO) that partners with biotech companies to accelerate the development of advanced and novel therapeutics at every phase, has released an expert report, Acute Myeloid Leukaemia - Global Clinical Trial Landscape, offering critical data-backed analysis of the latest developments in Acute Myeloid Leukemia (AML) research.
The report presents a comprehensive look at the AML funding landscape, trial density, patient recruitment data across geographies, standard of care, emerging therapies, recent USFDA approvals, and regulatory trends. Importantly it also includes an in-depth SWOT analysis to guide strategic decision-making, prioritize research areas, and identify challenges for clinical stage biotechs.
AML is a malignant condition affecting blood-forming stem cells, marked by disrupted differentiation and uncontrolled growth of myeloid lineages, and is the second most prevalent leukemia in both adults and children.
The Acute Myeloid Leukaemia (AML) - Global Clinical Trial Landscape report notes that since 2019, the global biotech and biopharmaceutical industry initiated over 1,000 clinical trials for AML.
The clinical trial data shows:
- North America leads at 40%
- Asia-Pacific is second at 33%
- Europe stands third at 22%
- ROW contributes a moderate share of 5%.
“In North America, the United States holds a majority trial share at 86%, while in Europe, Spain, France, Germany, and Italy lead AML trials, showing the region's contribution to advancing research. Within the Asia-Pacific region, China and Australia collectively represent the highest trial share (around 65%). Among the ROW countries, Israel takes the lead with 55% of AML trials,” the report found.
The report includes an examination of the various AML risk factors, including age, gender, chemical exposure, smoking, previous cancer treatments, and genetic predisposition.
It also highlights that chemotherapy is typically used in conjunction with targeted drug therapy to treat AML, however it finds that the treatment paradigm is shifting “to personalized approaches guided by molecular and genetic profiles and incorporating targeted therapies. The goal is to advance precision medicine for AML by understanding its evolving molecular complexities.”
The report states, “Clinical trial initiatives in precision oncology for AML are revolutionizing treatment approaches, driving transformative advancements, and enhancing patient outcomes.”
The incidence data shows key disparities across regions, with Asia claiming almost half of worldwide diagnoses at 46.6% (Europe 22.1%, North America 14.7% and ROW 16.6%). As such, measures have been taken amongst medical professionals in Asia to aid in early detection and support the increasing burden in the region.
- Asia reported over 68,000 AML cases, with Mainland China, India, and Japan contributing approximately 30% to the global incidence in the Asia-Pacific region.
- Mainland China alone accounted for over 35% of the reported AML cases in Asia.
- Europe documented over 32,000 AML cases, constituting over 20% of the global incidence.
- North American countries specifically the United States and Canada, together accounted for around 15% of the global incidence, with over 21,000 AML cases.
Additional key data covered in the report includes:
- The United States leads with venture capital funding ($1786.7m), followed by China ($1218.5m), with a large gap between countries following this.
- Among the leading companies that received funding for AML were Opna Bio SA, Shorla Oncology, CytosinLab Therapeutics Co. Ltd., and OncoVerity Inc.
- The emergence of molecular studies has revolutionized the diagnostic and therapeutic landscape of AML.
- FDA-approved molecular targeted drugs, including FLT3, IDH1, and IDH2 inhibitors, alongside mutation-independent agents like venetoclax and gemtuzumab ozogamicin, highlight the precision focus in AML management.
- AML drug development data shows 105 drugs in preclinical stages, 113 in Phase I, 104 in Phase II, and 22 in Phase III.
- Marketed drugs for AML display a diverse array of molecule types. Small molecules constitute the majority (>65%) of these drugs, while Proteins and Antibody-Drug Conjugates make up the remaining portion.
- Key opportunities identified were:
- Ongoing research focuses on developing new agents and immunotherapies with improved efficacy and reduced toxicity profiles.
- Identifying reliable biomarkers can further personalise treatment strategies and predict response to specific agents.
- Research on early detection methods could improve prognosis and treatment outcomes.
FDA drug approvals received for AML over the last two years include ivosidenib particularly for its use in combination with azacitidine to treat newly diagnosed AML with a susceptible IDH1 mutation. Olutasidenib capsules received approval specifically for relapsed or refractory AML cases with a susceptible IDH1 mutation. Approval for quizartinib was received for the treatment of newly diagnosed FLT3 ITD-positive AML in adults. Additionally, the LeukoStrat CDx FLT3 Mutation Assay also gained approval as a companion diagnostic for quizartinib.
The Novotech research analyst team provides these expert reports on a monthly basis, completely free of charge. These reports offer current insights into global clinical trial activity, revealing which regions experience the highest trial volumes and the unique factors behind these trends. They tackle the potential and real hurdles faced by biotech firms in specific therapeutic areas in the hopes to positively impact and inform clinical trial decision making, eventually improving rates of success with new treatments.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7825e3f7-01ff-4fa8-93c6-3eb8a863e82e
Media Contact
David James
mediacontact@novotech-cro.com
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427- instagram引流新方式:群发助手助你打破传统引流方法!
- 助力数字控糖,动脉网携手硅基仿生发布中国CGM真实应用首份蓝皮书
- FirstBank Puerto Rico Selects nCino to Transform Commercial Lending Services
- St Kitts and Nevis unveils the Investment Gateway Summit
- 第39届上海之春国际音乐节——上海小音乐家展演专场圆满落幕!
- 做完近视手术,医生都会跟你说这样一句话——福州爱尔眼科
- Gramercy Funds Management LLC任命新兴市场专家 Hamad Almudhaf为董事总经理兼国际资本市场主管
- 在野泽温泉体验真诚的健康关怀:通过中长期住宿促进健康旅游
- 全新 PICO® Nexμs™ 喷射系统助力流体点胶效率迈入工业 4.0 时代
- 2024年1月25日【泰州智车科技城】奠基暨企业入驻签约仪式盛大举行
- Junshi Biosciences Announces Approval of the sNDA for Toripalimab for the 1st-Line Treatment of Rena
- 尼尔森IQ胡姗姗出席福布斯中国美业峰会,全渠道新趋势助力品牌突围
- 内蒙古广达将军笑酒业有限公司:靠质量赢得市场 以诚信取得信任
- 品誉咨询——重痕迹→重实绩!挤水分,抓实效!
- 南京干冰厂干冰的主要用途干冰有什么用
- 运营线下陪玩平台,引流和复购,究竟哪个跟重要?
- 坚持创新驱动,澳柯玛以新质生产力推动产业发展
- 春节宅家煲剧首选!神剧《万物生灵》第三季欢喜首映独播上线治愈回归
- Liquid State全新力作,歌手周深带来春日重磅歌曲《春雪》
- 九运丹茶叶:醇香岁月,禅茶一味——南平市建阳区九运丹茶叶有限公司诠释国茶魅力
推荐
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
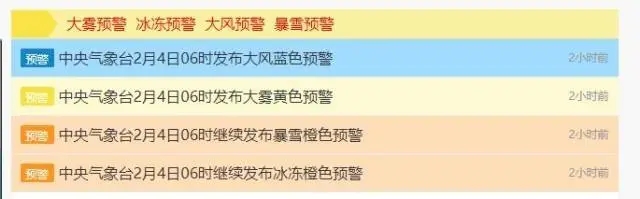 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
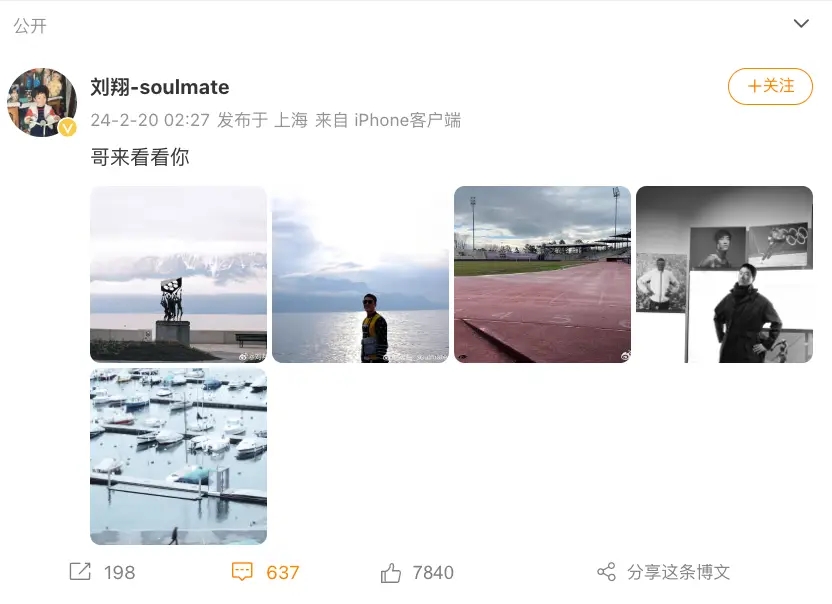 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯


