AIM ImmunoTech Announces Positive Top-Line, Protocol-Planned Interim Report Data from the Study of A
Robert Edwards, MD, Chair of the Department of Obstetrics, Gynecology & Reproductive Sciences and Co-Director of Gynecologic Oncology Research at Magee-Womens Hospital of UPMC
Robert Edwards, MD, Chair of the Department of Obstetrics, Gynecology & Reproductive Sciences and Co-Director of Gynecologic Oncology Research at Magee-Womens Hospital of UPMC
OCALA, Fla., April 10, 2024 (GLOBE NEWSWIRE) - AIM ImmunoTech Inc. (NYSE American: AIM) (“AIM”) today announced top-line interim data indicating that combining Ampligen (rintatolimod) with Keytruda (pembrolizumab) in the treatment of recurrent ovarian cancer may have a powerful synergistic effect, leading the investigator to conclude that the combination therapy could be far more effective than pembrolizumab alone as a therapy for the disease.
See further details on the study “Systemic Immune Checkpoint Blockade and Intraperitoneal Chemo-Immunotherapy in Recurrent Ovarian Cancer” at ClinicalTrials.gov: NCT03734692. Additionally, the immunological signature supporting this synergistic enhancement has been seen in other clinical trials, including with pancreatic cancer (1,2) metastatic triple-negative breast cancer and colorectal cancer metastatic to the liver.
Ampligen is a dsRNA product candidate that acts via the TLR-3 receptor present on several immune cells, epithelial cells and most solid tumors. In the ongoing, investigator-initiated Phase 2, single-arm efficacy/safety trial, University of Pittsburgh Medical Center researchers saw an Objective Response Rate (“ORR”) of 45% when combining Ampligen, pembrolizumab and cisplatin in platinum-sensitive subjects with recurrent ovarian cancer. ORR includes complete response (“CR”) and partial response (“PR”) to treatment. There was a total Clinical Benefit Rate (“CBR”) of 55% when including patients who experienced stable disease (“SD”). Researchers also reported a median Progression-Free Survival (“PFS”) of 7.8 months.
Robert Edwards, MD, Chair of the Department of Obstetrics, Gynecology & Reproductive Sciences and Co-Director of Gynecologic Oncology Research at Magee-Womens Hospital of UPMC, stated: “These results are incredibly favorable when compared to data from the hallmark Phase 2 study Keynote-100, which looked at the use of pembrolizumab alone in the treatment of recurrent ovarian cancer in both platinum-resistant and platinum-sensitive subjects. Keynote-100 reported an ORR of approximately 8% in these subjects – meaning that the new data analysis showed that combining pembrolizumab treatment with Ampligen created a greater than 500% increase in ORR over the Keynote-100 findings. Additionally, Keynote-100’s median PFS was 2.1 months, or significantly less than that seen in the ongoing Ampligen study. Additionally, the new ovarian cancer data analysis revealed an acute increase in anti-tumor immunity — specifically in biomarkers CXCL9, CXCL10, CXCL11 — which is consistent with the immune-stimulatory effects of Ampligen that researchers have seen in clinical studies of other solid tumors, including triple-negative breast cancer, pancreatic cancer and colorectal cancer. We look forward to publishing a more detailed analysis of these data in a peer-reviewed clinical journal this summer.”
AIM Chief Executive Officer Thomas K. Equels stated: “These interim data clearly suggest that there may be a massive positive impact on efficacy when Ampligen is combined with pembrolizumab for the treatment of recurrent ovarian cancer. Other research suggests a similar effect in other solid tumor types. We therefore see an Ampligen combination therapy as having potential across multiple types of cancers. We look forward to the additional clinical studies underway and planned in many of these types of tumors to further confirm these effects.”
About AIM ImmunoTech Inc.
AIM ImmunoTech Inc. is an immuno-pharma company focused on the research and development of therapeutics to treat multiple types of cancers, immune disorders and viral diseases, including COVID-19. The Company’s lead product is a first-in-class investigational drug called Ampligen® (rintatolimod), a dsRNA and highly selective TLR3 agonist immuno-modulator with broad spectrum activity in clinical trials for globally important cancers, viral diseases and disorders of the immune system.
For more information, please visit aimimmuno.com and connect with the Company on X, LinkedIn, and Facebook.
Cautionary Statement
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “continue,” “believe,” “potential,” “upcoming” and other variations thereon and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. Many of these forward-looking statements involve a number of risks and uncertainties. Publication of this data and clinical success seen to date does not guarantee that Ampligen will be approved for the commercial treatment of ovarian cancer. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the U.S. Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Among other things, for those statements, the Company claims the protection of the safe harbor for forward-looking statements contained in the PSLRA. The Company does not undertake to update any of these forward-looking statements to reflect events or circumstances that occur after the date hereof.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/1fd6cd3d-f807-45b8-87cb-2b30cf5cafba
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
AIM@jtcir.com- 北京春季购物逛街好去处:王府中环春季大牌穿搭攻略,打造时髦前卫造型
- 全球化战略范本:小鹏汽车正式进军港澳 香港最快第三季度开始交付
- 重庆润兴长途救护车出租转运服务中心,为患者提供舒适、安全、及更便捷的服务
- 让闺蜜先老 我要滤镜之谜的FILTERS滤镜之谜面膜究竟有何魅力
- 书法报书画天地·关注——旭宇
- LambdaTest将三星Galaxy S24系列纳入其真实设备云,以此引领市场潮流
- 春节出境游热度飙升 澳大利亚成为最受欢迎的长线旅游目的地
- 福能源好大夫入选中国特色视康行业特色加盟企业
- “同行十载·合力向新”银盛支付2024年合作伙伴大会·郑州站圆满举行
- 憨萌小狼温暖少女心灵,真事改编治愈电影《薇琪的秘密》欢喜首映独播上线
- 寻迹溯源相互制发展,中财保险学院师生走进信美相互人寿
- 青花釉里红玉壶春瓶:古韵今风,艺术珍品
- UN Global Compact launches Guidebook to encourage companies to Empower Women in the Workplace
- Banle Energy International Limited Supports BYD's Maiden Voyage of Car Carrier
- 共赴鸿蒙生态征途,凡泰极客入围华为开发者联盟生态市场服务商!
- 南方东英日经225指数ETF (3153.HK) 将于明日在香港交易所上市
- 千亿高活性趋势引领下的热销益生菌产品
- 赵今麦演绎连卡佛甄选MING MA西服套装
- Oceana: Future of returnable bottle packaging at risk following the sale of Coca-Coca Philippines
- 明星的宠儿“FILTERS滤镜之谜”面膜的背后究竟是什么?
推荐
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
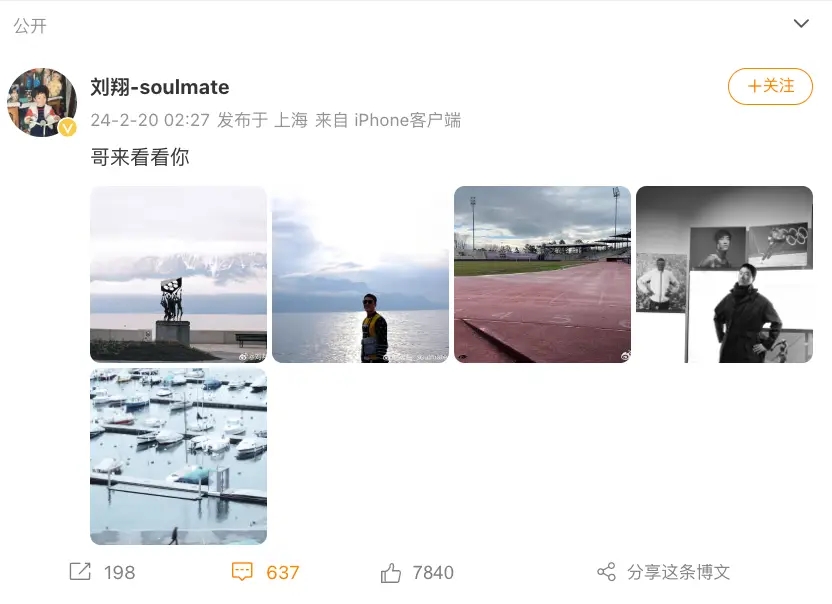 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯



