Nyxoah Reports First Quarter 2024 Financial and Operating Results
REGULATED INFORMATION
Nyxoah Reports First Quarter 2024 Financial and Operating Results
Announced DREAM U.S. pivotal study achieved primary endpoints
On track for FDA approval as early as end of 2024
Mont-Saint-Guibert, Belgium – May 14, 2024 10:05pm CET / 4:05pm ET – Nyxoah SA (Euronext Brussels/Nasdaq: NYXH) (“Nyxoah” or the “Company”), a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea (OSA), today reported financial and operating results for the first quarter of 2024.
Recent Financial and Operating Highlights
- Reported the DREAM U.S. pivotal study achieved co-primary endpoints on an intent-to-treat (ITT) basis and demonstrated strong AHI reductions in supine and non-supine sleep positions.
- The DREAM study achieved a median AHI reduction of 70.8%, a 12-month AHI responder rate, per the Sher criteria, of 63.5% (p=0.002) on an ITT basis and a 12-month ODI responder rate of 71.3% (p<0.001) on an ITT basis.
- Preparing the fourth and final PMA module for submission this quarter.
- Appointed Dr. Maurits S. Boon, MD as Chief Medical Officer.
- Advanced patient access strategy through partnership with the American Association of Otolaryngology – Head & Neck Surgery Foundation (AAO-HNSF).
- Achieved quarterly sales of €1.2 million, showing 170% growth vs Q1 2023.
- Total cash position of €44.3 million at the end of the quarter.
“The DREAM U.S. study achieving its primary endpoints is a pivotal milestone for Nyxoah and further differentiates Genio as the only HGNS therapy to demonstrate strong efficacy in supine and non-supine OSA. With the DREAM data in hand, our U.S. launch preparations are focused on attracting commercial talent to set us up for success when we introduce Genio,” commented Olivier Taelman, Nyxoah Chief Executive Officer. “With continued European commercial traction, positive DREAM data and a highly differentiated, patient centric HGNS solution, I could not be more excited for Nyxoah’s future.”
First Quarter 2024 Results
CONSOLIDATED STATEMENTS OF LOSS AND OTHER COMPREHENSIVE LOSS (unaudited)
(in thousands)
| For the three months ended March 31, | |||
| 2024 | 2023 | ||
| Revenue | € 1,221 | € 441 | |
| Cost of goods sold | (455) | (175) | |
| Gross profit | € 766 | € 266 | |
| Research and Development Expense | (7,199) | (6,157) | |
| Selling, General and Administrative Expense | (5,972) | (5,551) | |
| Other income/(expense) | 192 | 46 | |
| Operating loss for the period | € (12,213) | € (11,396) | |
| Financial income | 1 408 | 625 | |
| Financial expense | ( 991) | ( 958) | |
| Loss for the period before taxes | € (11,796) | € (11,729) | |
| Income taxes | ( 110) | ( 182) | |
| Loss for the period | € (11,906) | € (11,911) | |
| Loss attributable to equity holders | € (11,906) | € (11,911) | |
| Other comprehensive income/(loss) | |||
| Items that may not be subsequently reclassified to profit or loss (net of tax) | |||
| Currency translation differences | 60 | (28) | |
| Total comprehensive loss for the year, net of tax | € (11,846) | € (11,939) | |
| Loss attributable to equity holders | € (11,846) | € (11,939) | |
| Basic loss per share (in EUR) | € (0.415) | € (0.460) | |
| Diluted loss per share (in EUR) | € (0.415) | € (0.460) | |
CONSOLIDATED STATEMENT OF FINANCIAL POSITION (unaudited)
(in thousands)
| As at | |||||
| March 31 2024 |
December 31 2023 | ||||
| ASSETS | |||||
| Non-current assets | |||||
| Property, plant and equipment | €4,379 | €4,188 | |||
| Intangible assets | 48,501 | 46,608 | |||
| Right of use assets | 3,597 | 3,788 | |||
| Deferred tax asset | 134 | 56 | |||
| Other long-term receivables | 1 333 | 1,166 | |||
| € 57,944 | € 55,806 | ||||
| Current assets | |||||
| Inventory | 3,418 | 3,315 | |||
| Trade receivables | 2,971 | 2,758 | |||
| Other receivables | 3,149 | 3,212 | |||
| Other current assets | 1,232 | 1,318 | |||
| Financial assets | 22,225 | 36,138 | |||
| Cash and cash equivalents | 22,077 | 21,610 | |||
| € 55,072 | € 68,351 | ||||
| Total assets | € 113,016 | € 124,157 | |||
| EQUITY AND LIABILITIES | |||||
| Capital and reserves | |||||
| Capital | 4,927 | 4,926 | |||
| Share premium | 246,188 | 246,127 | |||
| Share based payment reserve | 8,440 | 7,661 | |||
| Other comprehensive income | 197 | 137 | |||
| Retained loss | (172,555) | (160,829) | |||
| Total equity attributable to shareholders | € 87,197 | € 98,022 | |||
| LIABILITIES | |||||
| Non-current liabilities | |||||
| Financial debt | 8,616 | 8,373 | |||
| Lease liability | 2,933 | 3,116 | |||
| Pension liability | 22 | 9 | |||
| Provisions | 273 | 185 | |||
| Deferred tax liability | − | 9 | |||
| € 11,844 | € 11,692 | ||||
| Current liabilities | |||||
| Financial debt | 346 | 364 | |||
| Lease liability | 852 | 851 | |||
| Trade payables | 7,316 | 8,108 | |||
| Current tax liability | 2,091 | 1,988 | |||
| Other payables | 3,370 | 3,132 | |||
| € 13,975 | € 14,443 | ||||
| Total liabilities | € 25,819 | € 26,135 | |||
| Total equity and liabilities | € 113,016 | € 124,157 | |||
Revenue
Revenue was €1.2 million for the first quarter ending March 31, 2024, compared to €441,000 for the first quarter ending March 31, 2023. The increase in revenue was attributable to the Company’s commercialization of the Genio® system, primarily in Germany.
Cost of Goods Sold
Cost of goods sold was €455,000 for the three months ending March 31, 2024, representing a gross profit of €0.8 million, or gross margin of 62.7%. This compares to total cost of goods sold of €175,000 in the first quarter of 2023, for a gross profit of €266,000, or gross margin of 60.3%.
Research and Development
For the first quarter ending March 31, 2024, research and development expenses were €7.2 million, versus €6.2 million for the first quarter ending March 31, 2023.
Operating Loss
Total operating loss for the first quarter ending March 31, 2024 was €12.2 million versus €11.4 million in the first quarter ending March 31, 2023. This was driven by the acceleration in the Company’s R&D spending, as well as ongoing commercial and clinical activities.
Cash Position
As of March 31, 2024, cash and financial assets totaled €44.3 million, compared to €57.7 million on December 31, 2023. Total cash burn was approximately €4.5 million per month during the first quarter 2024.
First Quarter 2024
Nyxoah’s financial report for the first quarter 2024, including details of the consolidated results, are available on the investor page of Nyxoah’s website (https://investors.nyxoah.com/financials).
Conference call and webcast presentation
A webcast of the call will be accessible via the Investor Relations page of the Nyxoah website or through this link: Nyxoah's Q1 2024 earnings call webcast. For those not planning to ask a question of management, the Company recommends listening via the webcast.
If you plan to ask a question, please use the following link: Nyxoah’s Q1 2024 earnings call. After registering, an email will be sent, including dial-in details and a unique conference call access code required to join the live call. To ensure you are connected prior to the beginning of the call, the Company suggests registering a minimum of 10 minutes before the start of the call.
About Nyxoah
Nyxoah is a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea (OSA). Nyxoah’s lead solution is the Genio® system, a patient-centered, leadless and battery-free hypoglossal neurostimulation therapy for OSA, the world’s most common sleep disordered breathing condition that is associated with increased mortality risk and cardiovascular comorbidities. Nyxoah is driven by the vision that OSA patients should enjoy restful nights and feel enabled to live their life to its fullest.
Following the successful completion of the BLAST OSA study, the Genio® system received its European CE Mark in 2019. Nyxoah completed two successful IPOs: on Euronext Brussels in September 2020 and NASDAQ in July 2021. Following the positive outcomes of the BETTER SLEEP study, Nyxoah received CE mark approval for the expansion of its therapeutic indications to Complete Concentric Collapse (CCC) patients, currently contraindicated in competitors’ therapy. Additionally, the Company is currently conducting the DREAM IDE pivotal study for FDA and US commercialization approval.
For more information, please visit http://www.nyxoah.com/.
Caution – CE marked since 2019. Investigational device in the United States. Limited by U.S. federal law to investigational use in the United States.
Forward-looking statements
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company's or, as appropriate, the Company directors' or managements' current expectations regarding the Genio® system; planned and ongoing clinical studies of the Genio® system; the potential advantages of the Genio® system; Nyxoah’s goals with respect to the development, regulatory pathway and potential use of the Genio® system; the utility of clinical data in potentially obtaining FDA approval of the Genio® system; and the Company's results of operations, financial condition, liquidity, performance, prospects, growth and strategies. By their nature, forward-looking statements involve a number of risks, uncertainties, assumptions and other factors that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties, assumptions and factors could adversely affect the outcome and financial effects of the plans and events described herein. Additionally, these risks and uncertainties include, but are not limited to, the risks and uncertainties set forth in the “Risk Factors” section of the Company’s Annual Report on Form 20-F for the year ended December 31, 2023, filed with the Securities and Exchange Commission (“SEC”) on March 20, 2024, and subsequent reports that the Company files with the SEC. A multitude of factors including, but not limited to, changes in demand, competition and technology, can cause actual events, performance or results to differ significantly from any anticipated development. Forward looking statements contained in this press release regarding past trends or activities are not guarantees of future performance and should not be taken as a representation that such trends or activities will continue in the future. In addition, even if actual results or developments are consistent with the forward-looking statements contained in this press release, those results or developments may not be indicative of results or developments in future periods. No representations and warranties are made as to the accuracy or fairness of such forward-looking statements. As a result, the Company expressly disclaims any obligation or undertaking to release any updates or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions or circumstances on which these forward-looking statements are based, except if specifically required to do so by law or regulation. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person's officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
Contacts:
Nyxoah
David DeMartino, Chief Strategy Officer
IR@nyxoah.com
- 辣味不简单!三养火辣酱再出奶油味新品让你“一蘸到底”
- 张慧雯《欢乐颂5》收官 演技受认可
- 捷报丨水星家纺荣膺“2023睡眠产业影响力企业”
- 储能节能、车位级运营 | 捷顺科技2024春季发布会推出多款行业新品新方案
- 中信银行太原分行开展“3.15”金融知识进社区宣传志愿活动
- 涓子再演《上错花轿嫁对郎》延续经典 新版《花轿喜事》角色反差大
- 天后容祖儿压轴华丽登场新濠尊属系列《容祖儿Eternity演唱会2024》 与歌迷约定共渡第二季新濠限定永恒梦幻音乐旅程
- 人气摄影比赛获奖者在大型新展览开幕之际公布,展现世界各地混凝土的重要作用和美感
- Curia 与 Carterra 合作举办生物制品专题研讨会,促进太平洋西北地区的生物技术研究
- Spatial宣布发布2024 1.0.1版
- 小金豆子们的挚爱,尔滨中式汉堡值得每一口的感动!
- Concentrix + Webhelp 重新确立品牌名为 Concentrix
- 奉贤经发大有界招商大会举办 一元股份携手上海国企打造区域商业新核
- WhatsApp协议注册软件,WS云控注册助你注册更简单!
- 明星的宠儿“FILTERS滤镜之谜”面膜的背后究竟是什么?
- 建设头痛防控基地及体系,提高偏头痛规范化诊断与治疗
- Perfect Moment 宣布首次公开募股结束
- 中国新加坡将互免签证,华人慈善家卓顺发太平绅士将推波助澜
- New Global Survey from Egon Zehnder Reveals the CFO Role is Bigger Than Ever, “Super CFOs” Continue
- 原来11位现在12位华人诺贝尔奖获得者3位代表中国获奖
- Boston Metal在巴西设立高价值金属生产子公司
- 初心如磐•向新出发 | 2024智能家居UP峰会暨CSHIA智能家居开年盛典成功举办
- 能敬资本梦想创业营参赛者张建斌-学到老活到老
- 荣登央视新闻联播,火星人集成灶智能制造肩负经济大梁
- 频频站上流量风口的招商雲墨,凭什么称为高新CID天花板?
- 好友团体验马术文化,《周游记2》探索诺曼底“双子城”
- Global Africa Business Initiative announces plans for Unstoppable Africa 2024 flagship event in New
- Andersen Global加强在中国的业务
- 只在课本上读过?五一来山海经奇,带你走进神秘的上古世界
- 绿色国际:共筑生态文明,开创绿色未来
推荐
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
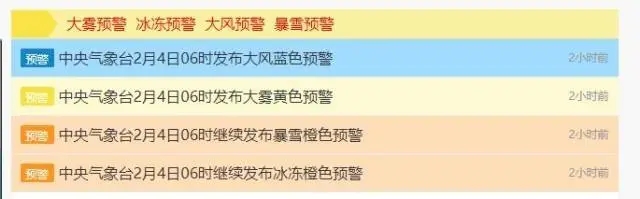 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
透过数据看城乡居民医保“含金量” 缴费标准是否合理?
记者从国家医保局了解到,近期,全国大部分地区
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯

