Novotech Liver Cancer Research Landscape Report Finds 1,700 Trials Initiated Globally
BOSTON, June 22, 2024 (GLOBE NEWSWIRE) -- Novotech, a global full-service clinical Contract Research Organization (CRO) specializing in partnering with biotech companies to expedite the development of cutting-edge therapeutics across all phases, has today published a leading industry report, Liver Cancer - Global Clinical Trial Landscape. Liver cancer is the sixth most diagnosed cancer worldwide and the third-most common cause of cancer-related deaths.
The report highlights the ongoing investment into liver cancer research globally and the significant treatment innovations that are designed to improve outcomes for patients.
The Novotech research analyst team provides these expert reports monthly, completely free of charge. These reports offer up-to-date insights into global clinical trial activity, revealing regions that experience the highest trial volumes and the factors behind these trends. They tackle the hurdles biotech firms face in specific therapeutic areas and discuss future paths in therapy and investment trends.
Hepatocellular carcinoma (HCC) is the predominant form of liver cancer, accounting for approximately 90% of all cases. HCC typically arises in individuals with chronic liver conditions such as hepatitis virus infection, cirrhosis, certain inherited liver diseases, or exposure to aflatoxins and affects men more than women.
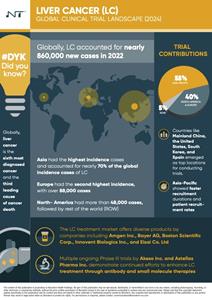
The Liver Cancer - Global Clinical Trial Landscape report finds that Asia had the highest incidence, contributing approximately 600,000 cases, which represented over 70% of the global burden. Within Asia, China alone reported more than 360,000 cases. Europe reported about 88,000 cases, while Africa faced significant challenges with 73,000 cases. North America saw over 48,000 cases, predominantly from the United States.
The Novotech report shows there’s a robust clinical trial pipeline for liver cancer, with over 1,700 trials initiated since 2018. Asia-Pacific and North America are at the forefront, contributing 55% and 24% respectively, followed by Europe at 16% and ROW at 5%, with Mainland China, South Korea, Spain and the United States emerging as top destinations for conducting trials.
These trials are across all phases, with a significant focus on early and mid-phase trials in Asia-Pacific and North America (Phase I and II), compared to Europe and the ROW which showed a lower overall number of trials. This data could suggest a difference in regional approaches to clinical research, possibly influenced by factors like funding, regulatory environments, and infrastructure.
Ongoing trials dominate globally, with Asia-Pacific leading again in ongoing and completed studies. North America and Europe closely follow, showing considerable activity in ongoing trials but fewer completed ones.
The treatment landscape for HCC is diverse and evolving. While surgical resection and liver transplantation offer the best long-term survival, many patients are unsuitable due to tumor size or liver conditions. Other treatments include traditional chemotherapy, targeted therapies like monoclonal antibodies and small molecules, and immunotherapies such as immune checkpoint inhibitors (ICIs) or tyrosine kinase inhibitors (TKIs). First-line treatments often combine atezolizumab and bevacizumab, while second-line options include lenvatinib, sorafenib, regorafenib, and cabozantinib.
Key takeaways from the report are:
- The drug development landscape includes approximately 70 drugs in preclinical stages, 23 in research, 51 in Phase I, 37 in Phase I/II, 66 in Phase II, 17 in Phase III, and 23 marketed drugs.
- Due to its large population and lower volume of studies, the Asia-Pacific region has lower competing trial risk with a trial density 4 times lower than that of the US and about 2 times lower than Europe.
- The funding landscape for liver cancer research shows substantial support, particularly through public and venture capital funding. The United States ($4,898 million) and China ($3,131 million) have seen significant venture capital investments in liver cancer research between 2019 and 2023.
- Funding predominantly supports preclinical, Phase I, and Phase II stages, with lesser amounts for discovery, Phase III, IND/CTA filed, and marketed stages.
- A SWOT analysis reveals strengths in multidisciplinary approaches and evolving therapies, but also highlights weaknesses like side effects and limited access to advanced treatments. Opportunities lie in precision medicine and immunotherapy, while threats include rising healthcare costs and insufficient funding for research.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f3310a56-88d7-4e4a-a0f5-8cc9bd2779ae
Media Contact
David James
mediacontact@novotech-cro.com
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427- 娄底瓷砖壁画/瓷砖烤字工厂【美佳壁画】
- GMAT™ Focus Edition Stands Alone in Business School Assessment as Previous Version Sunsets
- 财政部:分布式光伏发电将按电量补贴
- 看柠淘无人便利店如何将数据化运营与无感支付两者完美结合
- 奥运冠军推荐摩飞个护健康科技产品值得信赖
- 算力+存力:优化基因测序的关键所在
- 春季食养必备“神器”,一颗小红果筑起健康屏障
- RIB Software Cements Carbon Credentials with Groundbreaking 2050 Materials Partnership
- Instagram群发引流工具,ins批量私信软件,ig引流助手/Instagram协议号
- 解锁多模态智能新范式,趣丸科技AI应用斩获行业标杆案例
- 贵州融州华企业管理有限公司:专业化财税服务的新星
- Deutsche Telekom授予Mavenir云原生消息传递合同,推动欧洲四类网络的5G准备工作
- Music Licensing Inc Reports Strong Fiscal Year 2023 Results
- 抢鲜加码以旧换新 长虹美菱开启全国焕新行动
- MWC 2024 | 移远通信携手联发科技,率先打造5G CPE搭载Wi-Fi 7大规模商用案例
- “普利瓦科技(深圳)有限公司”受邀参加《大国时代》节目选题
- 【饮酒必备】步长青花瓷稳心颗粒可缓解饮酒前后的心跳加速
- 5个值得关注的家居原创品牌:栖作,梵几···
- 人民院线重磅首推!电影《你是我的英雄》定档5月20日热血上映
- 城市就是花园——北京花园城市高峰研讨会在京举办
- Recursion Announces Plans to Open New Office in London
- O泡时间到!童年的O泡又杀回来了
- 法国娇兰 艺术沙龙 全新香精系列
- 中医养生保健服务(非医疗)操作规范(推拿)首期培训结业
- 蒸烤炸一体机也成了减肥利器?一个月竟然减掉八斤肥肉
- 邦德薄荷咖啡入驻咖啡生活节,带来年轻人专属的“猫薄荷”
- 2024银川马拉松官方指定唯一乳制品赞助商——塞尚金河用生牛乳蛋白粉营养加持25000名跑者!
- Instagram自动化引流助手,ins协议号群发工具,ig营销神器/ins全参协议号
- 火星人集成灶以匠心售后服务构筑消费者权益坚实壁垒
- Instagram精准引流软件,ins协议号群发神器,ig群发软件/Instagram协议号
推荐
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯