Allecra Therapeutics and Acino Sign Exclusive Licensing and Supply Agreement for Allecra’s Novel Ant
Acino gains commercial rights for EXBLIFEP® (cefepime/enmetazobactam) within the member states of the Gulf Cooperation Council (GCC) and South Africa. EXBLIFEP® has been approved by the U.S. Food and Drug Administration (FDA) and the European Commission (EC) for the treatment of severe infections earlier in 2024.
Saint-Louis, France and Weil am Rhein, Germany and Zurich, Switzerland, June 24, 2024 (GLOBE NEWSWIRE) -- Allecra Therapeutics (“Allecra”) and Acino today announced the signing of an exclusive licensing agreement under which Acino gains the rights to commercialise Allecra’s antibiotic drug EXBLIFEP® (cefepime/enmetazobactam) within the Republic of South Africa and the member states of the GCC alliance, which includes Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates, effective from 12 June 2024. In addition, the companies have signed a supply agreement under which Allecra will supply the cefepime/enmetazobactam finished product in the above territories.

“Acino has established itself as a leader in South Africa and the GCC region. They are an ideal partner to support us as we build towards commercialisation of EXBLIFEP® following our regulatory approvals in the US and EU,” stated Andreas Kranzusch, Chief Financial Officer and Managing Director at Allecra Therapeutics. “This agreement reflects the understanding that there remains a significant global need to address the dangerous increase of resistance to standard-of-care antibiotics, and we look forward to working with Acino to address this.”
“At Acino, we are dedicated to providing novel healthcare solutions to physicians and patients, aiming to alleviate the health burden in emerging markets. We are incredibly excited to partner with Allecra to offer access to this innovative product in two key geographic regions and, potentially, beyond,” said Andrew Bird, CEO (ai) at Acino. “We are committed to expediting the registration process in these designated markets to ensure hospitals’ swift access to EXBLIFEP® as they continue to fight against high-risk infectious diseases in patients.”
About EXBLIFEP® (cefepime/enmetazobactam)
EXBLIFEP® is an intravenous antibiotic fixed-dose combination of enmetazobactam, a novel extended-spectrum β-lactamase inhibitor belonging to the penicillanic acid sulfone class, with the fourth-generation cephalosporin cefepime. Enmetazobactam has been shown to restore the efficacy of cefepime against some multi-drug resistant bacteria, including ESBL-producing pathogens alone or in combination with some resistant β-lactamase mutations as OXA-48 or AmpC, which are increasing in Europe and for which there are few therapeutic alternatives.
EXBLIFEP® demonstrated statistically significant superior overall treatment success in Allecra’s pivotal Phase III ALLIUM trial, which compared 1034 randomized patients receiving either cefepime 2 g/enmetazobactam 0.5 g or piperacillin 4 g/tazobactam 0.5 g every 8 h as 2 h continuous intravenous infusion in a multi-centre, randomized, controlled, double-blind, global study in 112 sites within nineteen countries.
In February 2024, the U.S. Food and Drug Administration (FDA) approved EXBLIFEP® as a treatment for complicated urinary tract infections (cUTI), including pyelonephritis, in patients 18 years and older. In March 2024 the European Commission (EC) granted marketing authorisation for EXBLIFEP® for the treatment of adult patients with cUTI, including pyelonephritis; hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP); and bacteraemia that occurs in association with, or is suspected to be associated with any of the infections listed previously.
About Allecra Therapeutics
Allecra Therapeutics, founded in 2013, is a private, clinical-stage biopharmaceutical company developing novel therapies to combat antibiotic resistance by overcoming emergent resistance mechanisms. Lead product candidate EXBLIFEP® (cefepime/enmetazobactam), has successfully completed a randomized, controlled, double-blind, global Phase 3 trial compared to standard of care in patients with complicated urinary tract infections (cUTIs). Based on these results, the company has received FDA marketing approval in the U.S. and announced approval in the European Union for EXBLIFEP® earlier this year.
Allecra has significant patent protection covering proprietary enmetazobactam in major territories. Allecra’s investors include Forbion, Andera Partners, Delos Capital, Xeraya Capital, EMBL Ventures, and BioMedPartners. Allecra’s wholly owned French subsidiary is a beneficiary of financial support from Bpifrance and the Région Alsace. Please visit www.allecra.com for further information.
About Acino
Acino is a Swiss pharmaceutical company headquartered in Zurich with a clear focus on selected markets in the Middle East, Africa, Ukraine, the CIS Region, and Latin America. We deliver quality pharmaceuticals to promote affordable healthcare in these emerging markets and leverage our high-quality pharmaceutical manufacturing capabilities and network to supply leading companies through contract manufacturing and out-licensing. For more information, please visit www.acino.swiss.
Acino is part of Arcera, a global company in the life sciences sector headquartered in Abu Dhabi, United Arab Emirates. Arcera was established by ADQ, an Abu Dhabi-based investment and holding company, to build a global life sciences powerhouse poised to make significant contributions to realising the UAE’s aspiration to emerge as a frontrunner in science and technology. To learn more about Arcera, please visit www.arceralifesciences.com.
For Allecra Therapeutics: Andreas Kranzusch, Chief Financial Officer and Managing Director ir@allecra.com Gretchen Schweitzer, Trophic Communications, +49 172 8618540 allecra@trophic.eu For Acino: Larisa Bernstein, Global Head of Communications larisa.bernstein@acino.swiss
- 大咖领航话青白,青年思辨求真知!第三届闽江白内障青光眼新技术培训班顺利举办
- 舒华体育亮相第83届中国教育装备展,校园智慧体育解决方案孕育未来栋梁
- 徐洁儿受邀惊艳出席2024星月榜样盛典 获“年度经典魅力演员”奖
- 天门瓷砖壁画瓷砖标识牌定制-美佳壁画
- 平安养老险深圳分公司携手福海街道开展5.12防灾减灾活动
- 2024中国湘商企业趋势发展论坛暨第九届大型湘商商务资源引荐会在莞成功举行
- Freshworks Announces Strategic Collaboration Agreement with AWS to Increase the Reach of its AI-boos
- 陈字酱带你来看白酒怎么选择
- Constellation Brands Appoints Sam Glaetzer to Lead Company’s Wine & Spirits Division
- 一抹细腻透亮,焕活青春无限 法国娇兰携手品牌护肤大使周翊然耀现年轻“蜂”采
- IBM+X-POWER+源卓微纳:以AI会友,共创制造业智能化故事2.0
- 呼吸道传染病(新冠、流感)混合高发,该如何选药?
- 金融领域神秘夫妇,资本市场上市公司内斗何时止息?
- DOCOMO与NEC将成立合资企业“OREX SAI”,为Open RAN的全球部署提供OREX Packages解决方案
- 蒙古政府和The Nature Conservancy确定“永恒蒙古”计划以实现蒙古气候和生物多样性目标
- 歌坛之神|邰上黄:歌坛生涯20年2024全新启航
- Gradiant旗下H+E赢得德国最大半导体晶圆厂之一的水处理设施建设合同
- Teledyne FLIR IIS扩展其Forge相机系列,达到IP67防护等级,适用于智能农业、食品和饮料行业
- 陈雨贤挑战全新风格 机车写真一展酷飒气质
- OPEN Health and fusion announce partnership to deliver AI-powered healthcare communications
推荐
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
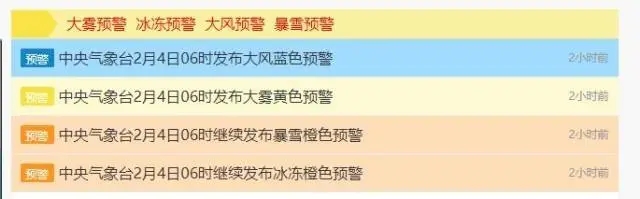 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
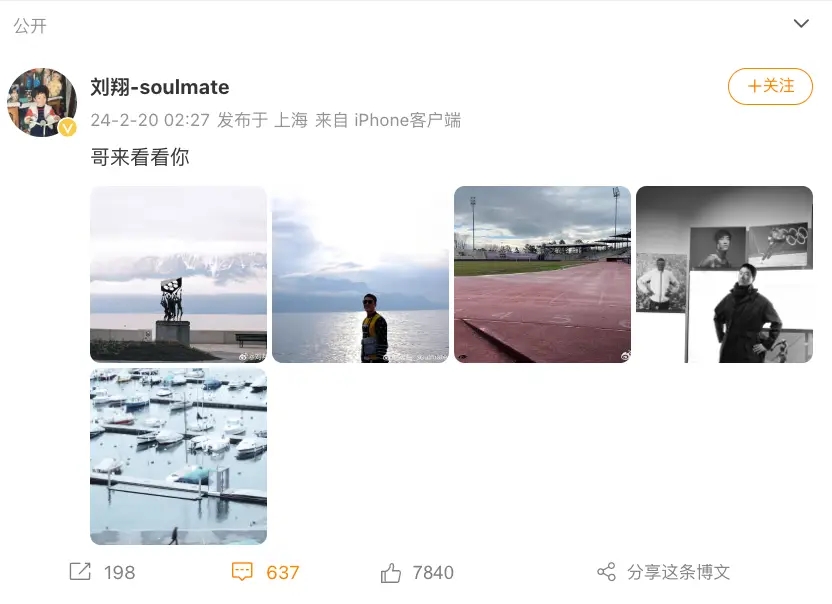 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯


