Sabin Sends More Marburg Vaccines for Rwanda’s Outbreak
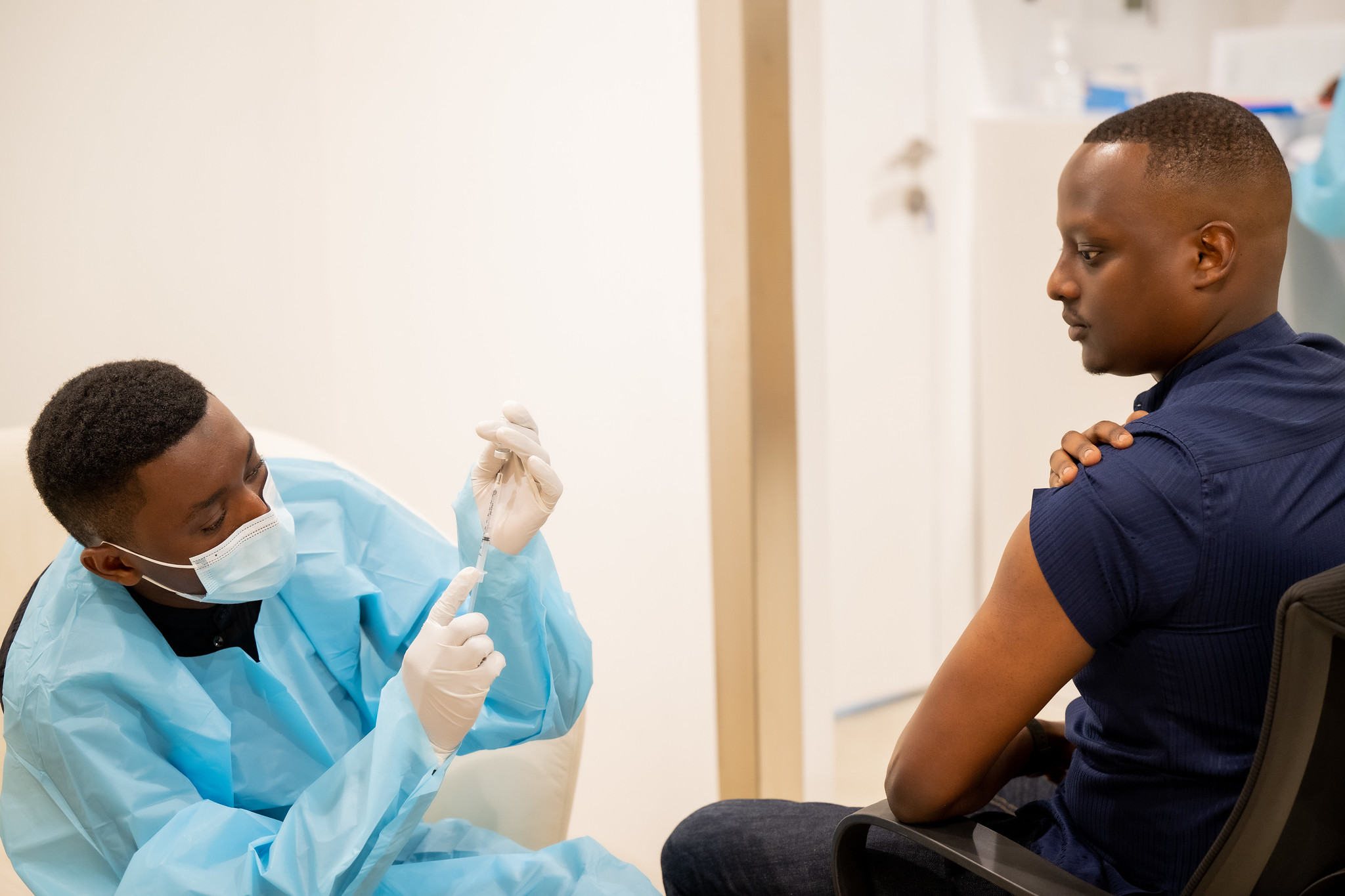
Photo caption: Rwanda Minister of State for Health, Yvan Butera, was among those who received Sabin’s investigational vaccine in the open-label clinical trial.
WASHINGTON, Oct. 31, 2024 (GLOBE NEWSWIRE) - In continued collaboration with Rwanda to address the Marburg virus outbreak, the Sabin Vaccine Institute has dispatched approximately 1,000 additional investigational vaccine doses for a randomized clinical trial arm within the ongoing open-label study. More than 1,500 frontline workers have already been vaccinated in Rwanda with the Sabin vaccine.
Under the updated protocol, sponsored by the Rwanda Biomedical Center, approximately 1,000 at-risk individuals, including mine workers, will receive Sabin’s single-dose investigational vaccine in a 1-to-1 randomization. Half will receive the vaccine immediately, and the other half 21 days later to align with the end of the disease incubation period.
Genomic sequencing of the index case (first identified case in an infectious disease outbreak) in Rwanda suggests a zoonotic origin, with strains similar to those found in fruit bats in a mine.
The new trial arm will assess safety, immunogenicity, and efficacy. Pending a request from Rwandan officials and authorization from the U.S. Administration for Strategic Response and Preparedness, Sabin plans to supply additional vaccines for this portion of the trial.
“As Rwandan health officials determined, the most expeditious and effective way to reach this new group of people impacted by the outbreak is to adapt the current protocol,” says Sabin CEO Amy Finan. “While this vaccine is still investigational, our mission is to ensure that knowledge can move swiftly from research to real-world solutions, with scientific rigor and safety as our highest priorities.”
At a news conference today about the Marburg outbreak, Rwanda minister of state for health Yvan Butera also addressed the need for more vaccine doses. Since the outbreak began, Sabin has been working directly with Rwandan officials and partners to mount a coordinated response.
As in previous outbreaks, Sabin continues to collaborate closely with its contract manufacturer, ReiThera, to prepare and ship the vialed doses to Rwanda.
Over 1,700 vaccines have already been delivered to Rwanda, with the first shipment of doses arriving just nine days after the outbreak was declared on September 27. The initial part of the trial focused largely on health workers, a group that suffered the most casualties in this outbreak.
Rwanda has confirmed 66 Marburg cases in what is one of the largest recorded outbreaks of the disease. Case numbers declined sharply within two weeks, with 15 deaths reported so far. The case-fatality rate, approximately 23%, remains significantly lower than previous outbreaks, which have seen mortality rates as high as 88%.
Designed to prevent illness before exposure to the virus, Sabin’s Marburg vaccine has not yet been proven to have clinical benefit for recipients of the vaccine. The candidate is currently in Phase 2 trials in Uganda and Kenya with no safety concerns reported to date. In non-human primates, it has demonstrated rapid immunity within one week, and in Phase 1 trials, it has shown safety and immunogenicity in humans.
Marburg virus disease remains a serious threat, with a high case fatality rate, and no approved vaccines currently available. Symptoms typically emerge between two and 21 days after infection.
Since 2019, Sabin has been at the forefront of advancing vaccines for filoviruses based on the cAd3 platform. Interim results from the Marburg trial are expected next year, with a U.S.-based trial planned for 2025. Sabin is also a key partner in MARVAC, a WHO-coordinated effort promoting global collaboration in Marburg vaccine development.
Sabin’s development program, which includes clinical trials and manufacturing of clinical trial material that have been leveraged for this current outbreak, is supported by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, under multi-year contracts. To date, BARDA has obligated $235 million to Sabin for advancing vaccine research and development against Sudan ebolavirus and Marburg virus diseases.
In addition to BARDA and Rwanda’s government, Sabin is grateful for all the organizations including CEPI, GSK, IQVIA, kENUP Africa, National Institutes of Health’s Vaccine Research Center, PPD, WHO, and World Courier who have contributed to our past and current efforts.
About the Sabin Vaccine Institute
The Sabin Vaccine Institute is a leading advocate for expanding vaccine access and uptake globally, advancing vaccine research and development, and amplifying vaccine knowledge and innovation. Unlocking the potential of vaccines through partnership, Sabin has built a robust ecosystem of funders, innovators, implementers, practitioners, policy makers and public stakeholders to advance its vision of a future free from preventable diseases. As a non-profit with three decades of experience, Sabin is committed to finding solutions that last and extending the full benefits of vaccines to all people, regardless of who they are or where they live. At Sabin, we believe in the power of vaccines to change the world. For more information, visit www.sabin.org and follow us on X, @SabinVaccine.
Media Contact:
Monika Guttman
Media Relations Specialist
Sabin Vaccine Institute
+1 (202) 662-1841
press@sabin.org
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f38d0939-533d-4fea-8d71-882be4461b28
- 上门解民忧 中信银行太原分行适老化金融服务提升温度
- 锦勇带您了解X射线辐射防护铅衣重要性,适用核能研究、科学实验
- 中秋国庆双节同欢乡村振兴专列同庆 农谷水寨品牌献礼双节助力乡村振兴
- IriusRisk partners with Adam Shostack to deliver threat modeling coaching services
- 2024年帝国大厦爬楼赛将10月9日回归
- DolphinDB 常见数据库错误代码大全
- 2024中国郑州新能源汽车生态伙伴大会暨智能网联汽车大赛隆重开幕
- 奥泰医疗荣膺“四川省首批标志性产品链主企业” 助力高性能医疗器械创新发展
- WD_BLCAK亮相核聚变游戏嘉年华 顶级存储赋能全场景游戏体验
- 农发行临澧县支行开展消防安全知识培训
- SLB宣布以全股票交易方式收购ChampionX
- 赵华为主演电影《维和防暴队》热映,初露锋芒迸发新人力量
- 王子璇领衔话剧《三姐妹》圆满成功,演绎经典作品绽放璀璨光芒
- 祝贺丨刘万鸣担任中国国家画院新一任院长
- telegram营销软件,精准定位海外需求客户低成本爆粉
- 降本增益成果显著 百利达集团2024年中期毛利率上升至22.1%
- Värde Promotes Shannon Gallagher and Tony Iannazzo to Partner
- 助力行业转型升级 浪潮海岳inSuite赋能冷链物流新“智”力
- 新品上市 | 日立厨房专用嵌入机,无惧油烟凉爽烹饪
- 《惜花芷》热播掀热潮,田淼张婧仪母女情深引热议
- 品誉咨询——销售“无界”→打造多元化销售战略
- 片仔癀经销商福建省韩廷集团公司助力福州市三明商会大厦封顶庆典圆满成功
- 帝斯曼-芬美意举办其首届中国可持续发展论坛 携手合作伙伴共推产业链绿色转型
- IBM:能力出海和企业出海的数字化能力
- 莆田如何办理注册香港公司办理香港公司审计等流程指南
- 助推医疗行业数智转型,天翼云获评2024年中国医疗云IaaS+PaaS市场领导者!
- UN Global Compact launches Guidebook to encourage companies to Empower Women in the Workplace
- 农发行郴州市分行办实培训打磨“三合一”专员精干队伍
- Honda 2024中国摩博会发布新战略、新车型价格,畅享逛展新体验
- 中信国际电讯CPC AI+创新方案闪耀世界人工智能大会
推荐
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯

