Nyxoah Reports Fourth Quarter and Financial Year 2023 Financial and Operating Results
Nyxoah Reports Fourth Quarter and Financial Year 2023 Financial and Operating Results
Expect to report DREAM U.S. pivotal study efficacy and safety data by early April
Achieved record quarterly sales of €1.8 million
Mont-Saint-Guibert, Belgium – March 5, 2024 10:05pm CET / 4:05pm ET – Nyxoah SA (Euronext Brussels/Nasdaq: NYXH) (“Nyxoah” or the “Company”), a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea (OSA), today reported financial and operating results for the fourth quarter and financial year 2023.
Recent Financial and Operating Highlights
Filed the third out of four modules in the U.S. modular PMA submission.
Achieved quarterly sales of €1.8 million, representing sequential quarterly growth of 87% and increasing 42% year-over-year.
Achieved 2023 full year sales of €4.3 million, representing 41% year-over-year growth.
Ended the year with 48 active German accounts, up from 38 entering 2023.
Total cash position of €57.7 million at the end of 2023.
2024 Strategic Priorities
Complete patient follow up in the DREAM U.S. pivotal study and report efficacy and safety data by early April.
File the fourth and final module in the modular PMA submission.
Accelerate investments in the U.S. commercial organization in preparation for a late 2024 launch.
Complete enrollment in the ACCCESS complete concentric collapse (CCC) U.S. pivotal study.
Increase hypoglossal nerve stimulation (HGNS) market penetration and Genio market share in Europe.
“In 2023, we completed enrollment in our DREAM U.S. pivotal study, presented positive early DREAM efficacy and safety data, initiated enrollment in our ACCCESS U.S. pivotal study for complete concentric collapse and raised capital from both existing and new investors. These accomplishments strengthen our confidence for a transformational 2024.” commented Olivier Taelman, Nyxoah Chief Executive Officer. “We are excited to report DREAM data in the coming weeks, finalize the regulatory FDA submission and pave the way for bringing Genio® to patients in the U.S.”
Mr. Taelman continued, “Commercially in Europe, this quarter’s performance was the strongest in Nyxoah’s history driven by a targeted direct-to-consumer (DTC) effort and I applaud our European commercial team for their execution. I look forward to a further increase in therapy penetration from our partnership with ResMed in Germany.”
Fourth Quarter and Full Year 2023 Results
UNAUDITED CONDENSED CONSOLIDATED FINANCIAL INFORMATION – CONSOLIDATED STATEMENTS OF LOSS AND OTHER COMPREHENSIVE LOSS FOR THE THREE MONTHS AND YEARS ENDED DECEMBER 31, 2023 AND DECEMBER 31, 2022 (in thousands)

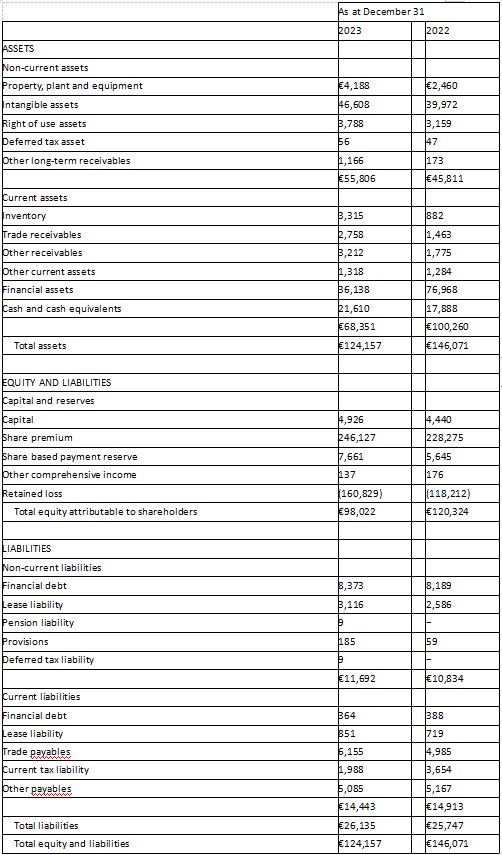
UNAUDITED CONDENSED CONSOLIDATED FINANCIAL INFORMATION – CONSOLIDATED STATEMENT OF FINANCIAL POSITION AS OF DECEMBER 31, 2023 AND DECEMBER 31, 2022 (in thousands)
Revenue
Revenue was €1.8 million for the fourth quarter ending December 31, 2023, compared to €1.3 million for the fourth quarter ending December 31, 2022. Revenue for the full year of 2023 was €4.3 million, compared to €3.1 million for the full year of 2022. The increase in revenue was attributable to the Company’s commercialization of the Genio® system, primarily in Germany.
Cost of Goods Sold
Cost of goods sold was €726,000 for the three months ending December 31, 2023, representing a gross profit of €1.1 million, or gross margin of 60%. This compares to total cost of goods sold of €465,000 in the fourth quarter of 2022, for a gross profit of €842,000, or gross margin of 64.4%.
For the full year ending December 31, 2023, total cost of goods sold was €1.7 million, representing a gross profit of €2.7 million, or gross margin of 62%. This compares to total cost of goods sold of €1.2 million for the full year of 2022, for a gross profit of €1.9 million, or gross margin of 62.7%.
Research and Development
For the full year ending December 31, 2023, research and development expenses were €26.7 million, versus €15.9 million for the full year of 2022.
Operating Loss
Total operating loss for the fourth quarter and full year 2023 was €10.8 million and €45.1 million, respectively, versus €9.1 million and €32.5 million in the fourth quarter and full year 2022, respectively. This was driven by the acceleration in the Company’s R&D spending, as well as ongoing commercial and clinical activities.
Cash Position
As of December 31, 2023, cash and financial assets totaled €57.7 million, compared to €94.9 million on December 31, 2022. Total cash burn was approximately €4.6 million per month during 2023.
Full year report 2023
Our independent auditor has not yet completed the audit of the financial statements for the year ended December 31, 2023. When published, the Nyxoah financial report for the full year of 2023 will be available on the investor page of Nyxoah’s website (https://investors.nyxoah.com/financials).
Conference call and webcast presentation
A webcast of the call will be accessible via the Investor Relations page of the Nyxoah website or through this link: Nyxoah's Q4 2023 earnings call webcast. For those not planning to ask a question of management, the Company recommends listening via the webcast.
If you plan to ask a question, please use the following link: Nyxoah’s Q4 2023 earnings call. After registering, an email will be sent, including dial-in details and a unique conference call access code required to join the live call. To ensure you are connected prior to the beginning of the call, the Company suggests registering a minimum of 10 minutes before the start of the call.
About Nyxoah
Nyxoah is a medical technology company focused on the development and commercialization of innovative solutions to treat Obstructive Sleep Apnea (OSA). Nyxoah’s lead solution is the Genio® system, a patient-centered, leadless and battery-free hypoglossal neurostimulation therapy for OSA, the world’s most common sleep disordered breathing condition that is associated with increased mortality risk and cardiovascular comorbidities. Nyxoah is driven by the vision that OSA patients should enjoy restful nights and feel enabled to live their life to its fullest.
Following the successful completion of the BLAST OSA study, the Genio® system received its European CE Mark in 2019. Nyxoah completed two successful IPOs: on Euronext Brussels in September 2020 and NASDAQ in July 2021. Following the positive outcomes of the BETTER SLEEP study, Nyxoah received CE mark approval for the expansion of its therapeutic indications to Complete Concentric Collapse (CCC) patients, currently contraindicated in competitors’ therapy. Additionally, the Company is currently conducting the DREAM IDE pivotal study for FDA and US commercialization approval.
For more information, please visit http://www.nyxoah.com/.
Caution – CE marked since 2019. Investigational device in the United States. Limited by U.S. federal law to investigational use in the United States.
Forward-looking statements
Certain statements, beliefs and opinions in this press release are forward-looking, which reflect the Company's or, as appropriate, the Company directors' or managements' current expectations regarding the Genio® system; planned and ongoing clinical studies of the Genio® system; the potential advantages of the Genio® system; Nyxoah’s goals with respect to the development, regulatory pathway and potential use of the Genio® system; the utility of clinical data in potentially obtaining FDA approval of the Genio® system; and the Company's results of operations, financial condition, liquidity, performance, prospects, growth and strategies. By their nature, forward-looking statements involve a number of risks, uncertainties, assumptions and other factors that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. These risks, uncertainties, assumptions and factors could adversely affect the outcome and financial effects of the plans and events described herein. Additionally, these risks and uncertainties include, but are not limited to, the risks and uncertainties set forth in the “Risk Factors” section of the Company’s Annual Report on Form 20-F for the year ended December 31, 2022, filed with the Securities and Exchange Commission (“SEC”) on March 22, 2023, and subsequent reports that the Company files with the SEC. A multitude of factors including, but not limited to, changes in demand, competition and technology, can cause actual events, performance or results to differ significantly from any anticipated development. Forward looking statements contained in this press release regarding past trends or activities are not guarantees of future performance and should not be taken as a representation that such trends or activities will continue in the future. In addition, even if actual results or developments are consistent with the forward-looking statements contained in this press release, those results or developments may not be indicative of results or developments in future periods. No representations and warranties are made as to the accuracy or fairness of such forward-looking statements. As a result, the Company expressly disclaims any obligation or undertaking to release any updates or revisions to any forward-looking statements in this press release as a result of any change in expectations or any change in events, conditions, assumptions or circumstances on which these forward-looking statements are based, except if specifically required to do so by law or regulation. Neither the Company nor its advisers or representatives nor any of its subsidiary undertakings or any such person's officers or employees guarantees that the assumptions underlying such forward-looking statements are free from errors nor does either accept any responsibility for the future accuracy of the forward-looking statements contained in this press release or the actual occurrence of the forecasted developments. You should not place undue reliance on forward-looking statements, which speak only as of the date of this press release.
Contacts:
Nyxoah
David DeMartino, Chief Strategy Officer
david.demartino@nyxoah.com
+1 310 310 1313
Attachment
ENGLISH_Q4 2023 Earnings PR_FINAL
- Lenovo在MWC 2024上发布旨在为“Power AI for All”愿景赋能的开拓性产品和解决方案
- SHEIN将通过直播时装秀“SHEIN Live: Front Row”发布2024春夏系列
- 海南明圣家具有限公司:传承匠心,打造品质国货品牌
- 喜讯!大明山感仙茶厂入选品牌强国优选工程茶行业典范企业
- 东莞市明辉新材料,诚信立户,品质兴业,一切为了客户需要!
- Nyxoah Announces 2024 Strategic Priorities
- 首届湾区罕见病早筛早诊早治大会暨第十七届世界罕见病日科普宣传活动圆满落幕!
- Laserfiche 渠道领导地位获得 2024 年 CRN®渠道领袖名单认可
- PUBG三月开春派对开启 丰厚奖励等你来拿
- 甲辰龙年已至 肯德基金桶盛满家乡年味儿送福到
- Origin Selects Fluence to Deliver the 300 MW / 650 MWh Mortlake Power Station Battery in Australia
- 中信银行渭南分行助力区域经济高质发展
- 平安养老险山西分公司:保护个人账户安全,切勿落入新型洗钱陷阱!
- 农发行郴州市分行开展 “三八”国际妇女节系列主题活动
- Pomilio Blumm: Italian Agency Claims Top Spot in FT's Growth Rankings
- 国际评级机构确认各Adani Portfolio公司评级并将展望升级为“稳定”
- 权威认可!美创再次入选CNCERT网络安全应急服务支撑单位
- 第十二届水业论坛召开,威派格引领智慧水务创新实践
- 2024年成都熠翊发服饰有限公司童装集合店成行业中的宠儿
- 圣雅菲与森纬升牵手合作,共创美丽未来
- Tecnotree在北美、中东、非洲和拉美地区成功为多个集团同时完成14次人工智能和数字化转型
- AI大佬点名,大摩看多,宁德时代领涨“AI尽头”
- Sanborn Map Company
- 全球引粉instagram群发引流软件:精准社交互动爆粉工具
- Philips extends leadership in virtually helium-free MRI with more than 1000 systems installed expand
- 春节再刷《狗剩快跑》,发现最稳健的演员是李梦男的“王大举”
- 喻问对话|纯良角码营销总经理周凤如:要为更多的门窗企业提供更高质的产品解决方案
- 你的年度时尚美妆关键词是?
- Saga与MARBLEX结成战略合作伙伴关系,共同推进Web3游戏的开发与应用
推荐
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
周星驰新片《少林女足》在台湾省举办海选,吸引了不少素人和足球爱好者前来参加
周星驰新片《少林女足》在台湾省举办海选,吸
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯

