Zymeworks to Present Preclinical Data on T cell Engager and Antibody-Drug Conjugate Platforms in Six

Novel trispecific T cell engager, ZW209, demonstrates potent preclinical efficacy against DLL3-expressing tumors and an encouraging safety profile
New antibody-drug conjugate (ADC) candidate, ZW327, exhibits anti-tumor activity and a favorable pharmacokinetics profile in Ly6E-bearing cancers
VANCOUVER, British Columbia, March 25, 2025 (GLOBE NEWSWIRE) -- Zymeworks Inc. (Nasdaq: ZYME), a clinical-stage biotechnology company developing a diverse pipeline of novel, multifunctional biotherapeutics to improve the standard of care for difficult-to-treat diseases, including cancer, inflammation, and autoimmune disease, today announced the acceptance of six abstracts for poster presentation at the upcoming American Association for Cancer Research (AACR) Annual Meeting being held April 24-30, 2025 in Chicago, IL.
“As our wholly-owned pipeline continues to progress, we are excited to share updates at AACR on our novel multifunctional therapeutics addressing difficult-to-treat cancers,” said Paul Moore, Ph.D., Chief Scientific Officer at Zymeworks. “Among these, we are excited to share updates on ZW209, our DLL3-targeting trispecific T cell engager incorporating co-stimulation, which is on track for IND submission in 1H-2026, and ZW327, our Ly6E-targeting antibody-drug conjugate incorporating our proprietary topoisomerase 1 inhibitor payload, ZD06519. These preclinical findings demonstrate how our therapeutic approaches have the potential to improve outcomes for patients with currently limited treatment options.”
Poster Presentation Details
T cell Engagers:
Title: ZW171, a differentiated 2+1 T cell-engaging bispecific antibody with antitumor activity in a range of mesothelin expressing cancers
Abstract: 3503
Session Category: Immunology
Session Title: T Cell Engagers
ZW171, a mesothelin (MSLN)-targeting T cell engager, currently in global Phase 1 clinical studies, shows promising preclinical activity in an expanded range of MSLN-positive tumor models including patient derived organoid and/or xenograft models of ovarian, pancreatic, and non-small cell lung cancer. These new findings expand our understanding of ZW171's differentiated profile and application to treat cancer models, further supporting our active clinical program's goal of helping patients with multiple types of difficult-to-treat cancers.
Title: ZW209, a DLL3 targeted trispecific T cell engager with integrated CD28 co-stimulation, demonstrates safety and potent preclinical efficacy in models of small cell lung cancer
Abstract: 7318
Session Category: Immunology
Session Title: T Cell Engagers and Novel Antibody-Based Therapies
ZW209, a trispecific T cell engager designed to optimally co-engage CD3 and CD28 on T cells and DLL3 on tumor cells, demonstrates potent and differentiated anti-tumor activity in multiple models of small cell lung cancer relative to benchmark DLL3 targeting T cell engagers. ZW209 also displays a favorable pharmacokinetics and safety profile in non-human primates following repeat dosing supporting further development.
Antibody-Drug Conjugates:
Title: ZW327, a novel Ly6E-targeting antibody-drug conjugate bearing a topoisomerase 1 inhibitor payload
Abstract: 2874
Session Category: Experimental and Molecular Therapeutics
Session Title: Antibody-Based Cancer Therapeutics 2
ZW327, a potential first-in-class ADC targeting Ly6E, an antigen overexpressed in numerous tumor types including breast, lung, and digestive tract cancers, demonstrates promising preclinical activity, highlighting its potential as an innovative therapeutic approach. Utilization of a superior Ly6E binding and internalizing antibody with a proprietary topoisomerase 1 inhibitor payload, ZD06519, lends ZW327 a highly differentiated profile, with pronounced in vitro cytotoxicity against a panel of tumor cell line models, single administration tumor regression in multiple xenograft models, and a well-tolerated profile in species cross reactive preclinical toxicology.
Title: Design and development of biparatopic antibody-drug conjugates against protein tyrosine kinase 7
Abstract: 1565
Session Category: Experimental and Molecular Therapeutics
Session Title: Antibody-Based Cancer Therapeutics 1
Protein Tyrosine Kinase 7 (PTK7) over expression across multiple tumor types including breast, digestive tract, and lung cancers, makes it an attractive target for ADCs. To enable optimal targeting, and overcome limitations of prior PTK7 ADCs, we have identified a lead biparatopic antibody displaying improved binding and internalization relative to that achieved with monospecific PTK7 antibodies. Relative to cofetuzumab pelidotin, a prior clinical stage PTK7 ADC, the lead PTK7 biparatopic antibody evaluated as an ADC utilizing Zymeworks’ proprietary topoisomerase 1 inhibitor payload, ZD06519, demonstrates increased efficacy in lung cancer xenograft models.
Zymeworks scientists are coauthors on two additional presentations leveraging technologies to aid further in design and characterizations of ADCs:
Title: High throughput quantitative molecular characterization of cytotoxic antibody-drug conjugates in spheroid models for improved functional characterization, screening and candidate selection
Abstract: 1230
Session Category: Tumor Biology
Session Title: 3D Models and Bioprinting
Title: In vitro assays for prediction of ADC hematological toxicity: contribution of antibody, linker, and payload
Abstract: 5482
Session Category: Experimental and Molecular Therapeutics
Session Title: Drug Discovery Assay Technologies
About Zymeworks Inc.
Zymeworks is a global clinical-stage biotechnology company committed to the discovery, development, and commercialization of novel, multifunctional biotherapeutics. Zymeworks’ mission is to make a meaningful difference in the lives of people impacted by difficult-to-treat conditions such as cancer, inflammation, and autoimmune disease. The Company’s complementary therapeutic platforms and fully integrated drug development engine provide the flexibility and compatibility to precisely engineer and develop highly differentiated antibody-based therapeutic candidates. Zymeworks engineered and developed zanidatamab, a HER2-targeted bispecific antibody using the Company’s proprietary Azymetric™ technology. Zymeworks has entered into separate agreements with BeiGene, Ltd. (BeiGene) and Jazz Pharmaceuticals Ireland Limited (Jazz Pharmaceuticals), granting each exclusive rights to develop and commercialize zanidatamab in different territories. The U.S. FDA granted accelerated approval of Ziihera® (zanidatamab-hrii) 50mg/mL for injection for intravenous use for the treatment of adults with previously-treated, unresectable or metastatic HER2-positive (IHC 3+) second-line biliary tract cancer (BTC). Ziihera® is the first and only dual HER2-targeted bispecific antibody approved for HER2-positive BTC in the U.S. Zanidatamab is currently under regulatory review in the EU and China for second-line BTC and is being evaluated in multiple global clinical trials as a potential best-in-class treatment for patients with multiple HER2-expressing cancers. Zymeworks is rapidly advancing a robust pipeline of wholly-owned product candidates, leveraging its expertise in both antibody-drug conjugates and multispecific antibody therapeutics targeting novel pathways in areas of significant unmet medical need. Phase 1 studies for ZW171 and ZW191 are now actively recruiting with an investigational new drug application for ZW251 planned for mid-2025. In addition to Zymeworks’ pipeline, its therapeutic platforms have been further leveraged through strategic partnerships with global biopharmaceutical companies. For information about Zymeworks, visit www.zymeworks.com and follow @ZymeworksInc on X.
Cautionary Note Regarding Forward-Looking Statements
This press release includes “forward-looking statements” or information within the meaning of the applicable securities legislation, including Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements in this press release include, but are not limited to, statements that relate to Zymeworks’ preclinical pipeline; the potential therapeutic effects of and commercial potential of zanidatamab and Zymeworks’ other product candidates; anticipated IND submissions and the timing thereof; Zymeworks’ clinical development of its product candidates and enrollment in its clinical trials; anticipated preclinical and clinical data presentations; the ability to advance product candidates into later stages of development; and other information that is not historical information. When used herein, words such as “plan”, “believe”, “expect”, “may”, “anticipate”, “potential”, “will”, “on track”, “continues”, and similar expressions are intended to identify forward-looking statements. In addition, any statements or information that refer to expectations, beliefs, plans, projections, objectives, performance or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking. All forward-looking statements are based upon Zymeworks’ current expectations and various assumptions. Zymeworks believes there is a reasonable basis for its expectations and beliefs, but they are inherently uncertain. Zymeworks may not realize its expectations, and its beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements as a result of various factors, including, without limitation: clinical trials may not demonstrate safety and efficacy of any of Zymeworks’ or its collaborators’ product candidates; any of Zymeworks’ or its partners’ product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; regulatory agencies may impose additional requirements or delay the initiation of clinical trials; the impact of new or changing laws and regulations; market conditions; inability to maintain or enter into new partnerships or strategic collaborations; and the factors described under “Risk Factors” in Zymeworks’ quarterly and annual reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K for its year ended December 31, 2024 (a copy of which may be obtained at www.sec.gov and www.sedarplus.ca).
Although Zymeworks believes that such forward-looking statements are reasonable, there can be no assurance they will prove to be correct. Investors should not place undue reliance on forward-looking statements. The above assumptions, risks and uncertainties are not exhaustive. Forward-looking statements are made as of the date hereof and, except as may be required by law, Zymeworks undertakes no obligation to update, republish, or revise any forward-looking statements to reflect new information, future events or circumstances, or to reflect the occurrences of unanticipated events.
Contacts:
Investor Inquiries:
Shrinal Inamdar
Senior Director, Investor Relations
(604) 678-1388
ir@zymeworks.com
Media Inquiries:
Diana Papove
Senior Director, Corporate Communications
(604) 678-1388
media@zymeworks.com
- 炬芯科技的智能手表SoC采用了芯原的2.5D GPU IP
- AVEVA通过DNV数字基础设施验证加速海事与海洋解决方案数字化
- 中国石油浙江台州销售分公司:第二党支部开展学雷锋日帮扶活动
- VCI Global Secures US$12 Million Contract With Datanex for AI-Powered Digital Marketing Solutions Th
- 2024年澳新投资年会于前滩中心圆满落幕
- DALI Alliance lighting awards opens with new categories for 2024
- 自动巡查昼夜待命,浙江打造消防无人机巡逻新模式
- Tigo Energy欢迎Zerun成为最新的快速关机许可方
- 应对甲流,奥司他韦和宣肺败毒颗粒联用可精准对敌!
- 日本清酒与烧酒制造商协会公布2024年清酒出口数据:出口至80个国家,同比增长6%,创下历史新高
- 上海礼智信影视文化传媒出品的《中华五千年.同福客栈》正式开拍
- 共赢未来!创维光伏E企赢模式加速企业绿色低碳转型步伐
- 远程商用车与国能集团内蒙古电力、爱德曼氢能源战略合作
- Italian Tradition Brings Flavour to Asia with ‘European Art of Taste
- Fortrea 推出 AI 创新工作室以整合技术和人力解决方案,改进临床试验交付
- Snail’s Innovates Development Pipeline with AI Technology
- 中国电能表平台火热招商中!!!
- WS科技商业的魔法时刻:WhatsApp工具,科技魔法师揭示市场趋势的奇异秘密
- 飞鹤奶粉第七次荣登“中国500最具价值品牌”榜单
- 沙特全民体育联合会公布首届SFA国际体育赛事的利雅得马拉松路线、场馆及赞助商
- C.K. McWhorter & Single Family Office to Pioneer Integration of Quantum Computing Technologies A
- 《邓婷访谈》纽约专访华裔精英、国际海南总商会创始人王黎娜女士
- 世贸通美国EB5投资移民:留学美国容易留在美国难,怎么办?
- 永和豆浆联合小白心里软 定义早餐新方式
- 都市爱情剧《难哄》今日开播 新人演员苏棋崭露头角引期待
- Avance Clinical 任命亚洲区域总监,作为全球扩展计划的一部分
- 中嘉裕福:为大学生打通求职“最后一公里”路
- 青春不散场,篮球永不止步——《左手上篮2》热血回归
- 权威媒体聚焦美业教育先锋:邓锋校长专访引领行业新风向
- 中信银行落地首批“全国中小微企业资金流信用信息共享平台”普惠贷款业务
推荐
-
 中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
中国减排方案比西方更有优势
如今,人为造成的全球变暖是每个人都关注的问
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
海南大学生返校机票贵 有什么好的解决办法吗?
近日,有网友在“人民网领导留言板&rdqu
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
产业数字化 为何需要一朵实体云?
改革开放前,国内供应链主要依靠指标拉动,其逻
资讯
-
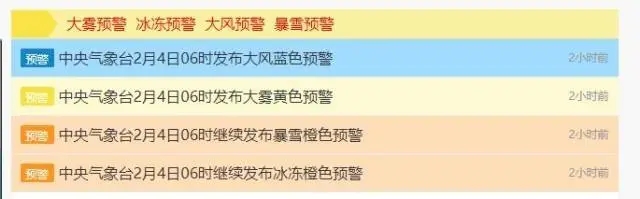 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
-
 新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
新增供热能力3200万平方米 新疆最大热电联产项目开工
昨天(26日),新疆最大的热电联产项目—&md
资讯
-
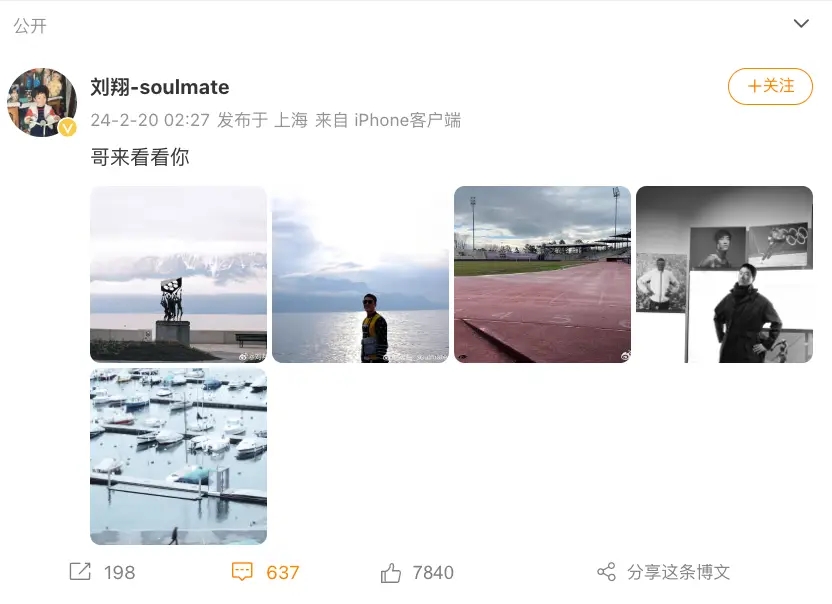 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯

