APRINOIA Therapeutics Announces Fast Track Designation Granted by U.S. FDA to APN-1607 for the Diagn
Fast Track Designation follows announcement in January 2024 that the U.S. Food and Drug Administration (FDA) granted a “Study May Proceed” letter for APRINOIA’s planned Phase 3 study of APN-1607 (florzolotau (18F)) in the U.S., U.K., Europe and Asia
CAMBRIDGE, Mass., May 21, 2024 (GLOBE NEWSWIRE) -- APRINOIA Therapeutics Inc. (“APRINOIA” or the “Company”), a clinical-stage biopharmaceutical company developing novel therapeutics and precision diagnostics for the treatment of neurodegenerative diseases, today announced that on May 8, 2024, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation (FTD) to APN-1607 (florzolotau (18F)), a Positron Emission Tomography (PET) tracer for imaging tau protein in patients with suspected progressive supranuclear palsy (PSP).
PSP is a rare neurodegenerative disorder caused primarily by the accumulation of a specific form of tau in subcortical brain regions. There are no FDA-approved diagnostic markers for PSP or any other rare tau-related disorder such as frontotemporal dementia, and until now, diagnosis has primarily relied on clinical assessment. APN-1607 may enable more accurate diagnosis at earlier disease stages, potentially improving patient management and resulting in more efficient clinical trial designs for novel therapies.
“We are very pleased with the FDA’s decision to grant APN-1607 Fast Track Designation as it underscores the significant unmet medical need for a diagnostic marker for the early diagnosis of PSP and potentially other tau-related disorders, including Alzheimer’s disease. APN-1607 is a unique imaging agent as it was designed to detect specific forms of tau implicated in PSP and other related disorders. Sadly, patients with PSP can remain undiagnosed for several years as it is often confused with other Parkinson’s like disorders, especially during the early stages. If approved, APN-1607 would provide physicians with an important diagnostic tool that will allow them to diagnose PSP with greater confidence and differentiate it from other disorders, thereby improving the management of these patients,” stated Dr. Brad Navia, Chief Medical Officer of APRINOIA Therapeutics.
“Receiving Fast Track Designation, along with the previously announced “Study May Proceed” letter from the FDA, reinforces the importance of our work related to APN-1607, and specifically, the clinical development plan to advance the asset into the clinic for the early diagnosis of PSP, and potentially other tau-related neurological disorders, including frontotemporal dementia and Alzheimer’s disease. We look forward to our further engagement with the FDA as a part of APN-1607’s Fast Track Designation, as we seek to accelerate the clinical development program for this important diagnostic tracer. We are grateful to our many investigators and partners, including the Alzheimer’s Drug Discovery Foundation and CurePSP for their continued support in our efforts to advance APN-1607 for the diagnosis of PSP and related disorders,” said Dr. Navia.
“Providing patients with neurodegenerative disorders, such as Alzheimer’s, and PSP with an early and accurate diagnosis is critical for determining the best course of treatment as well as accelerating drug development via clinical trials. The Fast-Track Designation for APRINOIA’s PET tracer is a milestone for the field that will serve as the first tau imaging agent for PSP and will add to the arsenal of tau imaging tools for Alzheimer’s. This new generation PET Tracer – in addition to other biomarkers – will move us closer to the day when we can treat the right patients with the right drugs at the right time through a precision medicine approach,” said Dr. Howard Fillit, Co-Founder and Chief Science Officer at the Alzheimer’s Drug Discovery Foundation (ADDF).
Fast Track Designation is designed to facilitate the development and expedite the review of product candidates that demonstrate the potential to address an unmet medical need, with the goal of advancing important new diagnostic and treatment options to patients more quickly than traditional regulatory routes.
Once a drug candidate receives Fast Track Designation, early and frequent communication with the FDA, including discussions around the product candidate’s development plan and regulatory review process are ensured. If the relevant criteria are met, the product candidate may be eligible for Accelerated Approval and Priority Review by the FDA.
About APN-1607
APN-1607 is a radioactive fluorinated molecule developed to visualize and quantify 3R and 4R tau aggregates by PET imaging across a number of diverse tau-related disorders, including PSP, frontotemporal dementia and Alzheimer’s disease, among others. APRINOIA is developing APN-1607 as a first-in-class radioactive diagnostic tracer for the detection of 3R and 4R tau aggregates, which contribute to the pathogenesis of various tau-related neurological disorders. APRINOIA previously received an Orphan Drug Designation from the FDA for APN-1607 as a diagnostic agent for PSP. APN-1607 has been clinically utilized in over 3,000 patients through investigator-initiated and sponsor trials, supporting its potential clinical utility as a diagnostic marker for tau-related disorders. In December 2023, enrollment of a Phase 3 trial to evaluate the efficacy and safety of APN-1607 for the diagnosis of Alzheimer’s disease was completed in China and on December 8, 2023, APRINOIA received a “Study May Proceed” letter from the U.S. FDA to conduct a global Phase 3, multicenter, open-label study to evaluate the efficacy and safety of APN-1607 as a diagnostic marker in patients suspected to have PSP.
About APRINOIA
APRINOIA Therapeutics Inc. is a clinical-stage biotechnology company, headquartered in Cambridge, MA, committed to developing highly sensitive and selective diagnostic and therapeutic agents for a broad range of neurodegenerative diseases. To learn more, please visit http://www.aprinoia.com and follow us on LinkedIn.
Forward-Looking Statement
This communication contains forward-looking statements which reflect APRINOIA’s current expectations regarding future events, including its expectations for the future development of the Company. APRINOIA’s actual results may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance, and underlying assumptions and other statements that are other than statements of historical facts. No representations or warranties, express or implied are given in, or in respect of, this communication. When APRINOIA uses words such as “may,” “will,” “intend,” “should,” “believe,” “expect,” “anticipate,” “project,” “estimate” or similar expressions that do not relate solely to historical matters, it is making forward-looking statements. Forward-looking statements speak only as of the date they are made and factors that may cause actual results to differ materially from current expectations include, but are not limited to: the performance of APRINOIA’s business; the risk that preclinical studies and early-stage clinical trials may not be predictive of future results; the risk that regulatory approvals for APRINOIA’s product development are not obtained or are delayed, and the timing, success and cost of APRINOIA’s pipeline development activities and clinical trials. There may be additional risks that APRINOIA does not presently know, or that APRINOIA currently believes are immaterial, that could cause actual results to differ from those contained in the forward-looking statements. APRINOIA undertakes no obligation to publicly revise these forward-looking statements to reflect events or circumstances that arise after the date of this communication, except as required by applicable law.
Contact
Nick Colangelo
IR@Aprinoia.com
- 平安养老险山西分公司:谨防假借私募基金经营 掩盖非法集资
- 慧商智慧:解读高慧商人的领导力秘笈!
- 浙江干冰仁裕干冰干冰清洗机干冰清洗服务
- 特安护品牌亮相太原煤炭展览会-展现中国个体防护民族品牌的良心智造
- St Kitts and Nevis Paves the Way for its Global Citizens to “Connect, Collaborate and Celebrate” at
- Smart Chain引领我们通往未来数字世界
- 重要成果丨洁特生物参与制定的细胞培养残留检测新国标发布!
- 智云健康数字化赋能药店经营提质增效
- Ambiq Apollo510 功耗效率提高 30 倍,释放终端 AI 潜能
- 热烈庆祝 吉林省融仨食品有限公司荣登CCTV-7广告展播
- Esteemed Excellence: C.K. McWhorter & McWhorter Family Trust Illuminates Aqua Panna Natural Mine
- 首批下线!“鲜活水”茶吧机重磅来袭,长虹美菱茶吧机迈入3.0时代
- 刘慈欣遇见薛定鹅科学,共绘中国科幻新篇章
- 根据Workiva的最新调查,可持续发展法规正在推动企业报告转型
- Ins引流新高度!Instagram自动聊天软件,多功能群发器震撼上线!
- 中国联通携手鱼跃医疗共建AED智能急救网络
- 科技创新之路:李国杰院士视角下的关键技术突破
- 2024中国当代百杰书画名家——石坤祖专题报道
- Cardano Foundation任命Giorgio Zinetti为首席技术官
- OBI Pharma Announces Poster Presentations at the AACR 2024 Annual Meeting for OBI-992 and GlycOBI™ A
- ResMed's 2024 Global Sleep Survey Uncovers a World in Sleep Crisis
- 送健康家庭智能高压萃取机LC1008植源灵萃
- 光子易PhotonPay受邀参展迪拜金融科技峰会,携全球一体化支付解决方案亮相
- 最新通报,奔驰车主插队砸车被拘10日
- 2024中国当代百杰书画名家——沈国良专题报道
- arena阿瑞娜正式宣布品牌代言人——徐嘉余
- 百胜中国打造美食新场景 活力四溢助力展会经济
- 杭州绍兴干冰清洗机干冰清洗技术块状干冰颗粒干冰食品级干冰
- 成都AG超玩会选手精准护眼要诀 优思益蓝莓护眼丸引爆关注
- 科技守护长久陪伴,火星人集成灶交上母亲节完美答卷
推荐
-
 私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
私域反哺公域一周带火一家店!
三四线城市奶茶品牌茶尖尖两年时间做到GMV
资讯
-
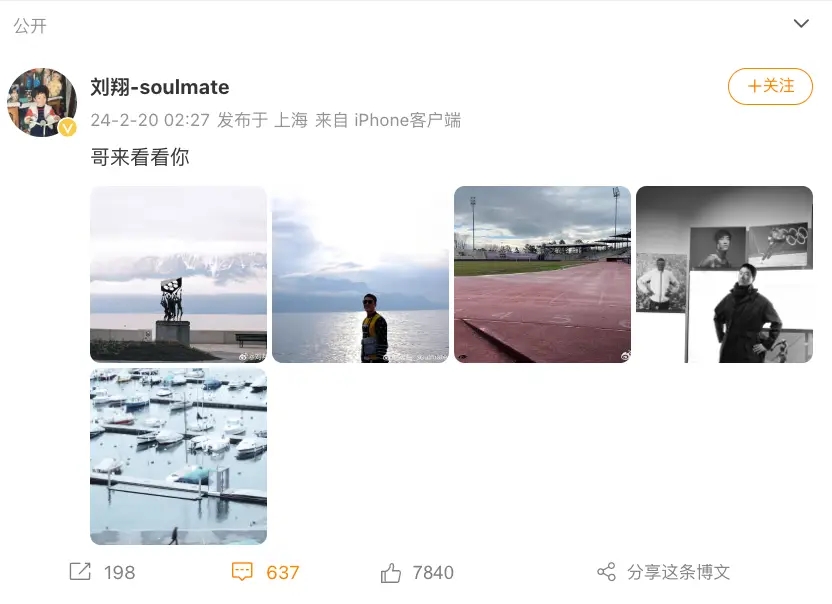 奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
奥运冠军刘翔更新社交账号晒出近照 时隔473天更新动态!
2月20日凌晨2点,奥运冠军刘翔更新社交账号晒
资讯
-
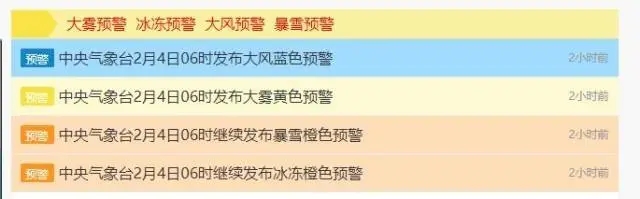 中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
中央气象台连发四则气象灾害预警
暴雪橙色预警+冰冻橙色预警+大雾黄色预警+
资讯
-
 抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
抖音直播“新红人”进攻本地生活领域
不难看出,抖音本地生活正借由直播向本地生活
资讯
-
 大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
大家一起关注新疆乌什7.1级地震救援见闻
看到热气腾腾的抓饭马上就要出锅、村里大家
资讯
-
 王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
王自如被强制执行3383万
据中国执行信息公开网消息,近期,王自如新增一
资讯
-
 看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
看新东方创始人俞敏洪如何回应董宇辉新号分流的?
(来源:中国证券报)
东方甄选净利润大幅下滑
资讯
-
 一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
一个“江浙沪人家的孩子已经不卷学习了”的新闻引发议论纷纷
星标★
来源:桌子的生活观(ID:zzdshg)
没
资讯
-
 男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
男子“机闹”后航班取消,同机旅客准备集体起诉
1月4日,一男子大闹飞机致航班取消的新闻登上
资讯
-
 国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯
国足13次出战亚洲杯首次小组赛0进球
北京时间1月23日消息,2023亚洲杯小组
资讯


